|
(11) | EP 0 000 323 B1 |
| (12) | EUROPEAN PATENT SPECIFICATION |
|
|
| (54) |
Deuterated 1,1-difluoro-2,2-dihaloethyl-difluoromethyl ethers, a process for their preparation and their use in anesthetics Deuterierte 1,1-Difluoro-2,2-dihaloäthyl-difluoromethyl Äther, ein Verfahren zu ihrer Herstellung und ihre Verwendung in anästhesierenden Mitteln Ethers 1,1-difluoro-2,2-dihalogéoéthyl-difluorométhyliques deutérés, un procédé pour leur préparation et leur utilisation dans des anesthésiques |
|
|
|||||||||||||||||||||||
| Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. Notice of opposition shall be filed in a written reasoned statement. It shall not be deemed to have been filed until the opposition fee has been paid. (Art. 99(1) European Patent Convention). |
[0001] This invention concerns deuterated. 1,1 - difluoro - 2,2 - dihaloethyl difluoromethyl ethers, use in an anesthetic, and a process for preparing the compounds.
[0002] Various 1,1 - difluoro - 2,2 - dihaloethyl difluoromethyl ethers have been described in the prior art and are known for use as inhalation anesthetics. A commonly used compound is 1,1,2 - trifluoro - 2 - chloroethyl difluormethyl ether also known as enflurane. Although the metabolic pathways of enflurane have not been defined, it is known the compound is metabolized in the body to produce inorganic fluorides in the blood which can cause renal dysfunction. In addition, elevated levels of serum bromides released from metabolized material containing bromine is responsible for post-anesthetic depression.
[0003] The present invention is directed to novel deuterated analogues of the known 1,1 - difluoro - 2,2 - dihaloethyl difluoromethyl ethers; the deuterated analogues have the general formula:
wherein X is fluoro and X' is bromo or chloro, or both X and X' are chloro.
[0004] The present invention is also directed to use of the compounds in an anesthetic, for example, a composition which comprises a compound of the present invention in combination with an anesthetically acceptable adjunct, particularly such a composition which has been formulated as an inhalant. By "adjunct" is meant to include both non-anesthetic diluents or carriers and other anesthetics, such as, for example, nitrous oxide. In anesthetizing an animal, the compound is usually adminstered by vaporizing the compound in the presence of an adjunct such as, for example, helium, nitrogen, oxygen, or various mixtures thereof. As used herein, the term "minimum alveolar concentration" refers to the effective concentration of the anesthetic or anesthetic combination required to produce the desired degree of anesthesia in the animal. The particular minimum alveolar concentration depends on factors well known in the art such as the animal to be anesthetized or the particular compound employed.
[0005] One method for preparing the 1,1 - difluoro - 2 - deutero - 2,2 - dihaloethyl difluoromethyl ethers that are the subject of the present invention is by a base catalyzed deuterium exchange involving the hydrogen atom in the 2 - ethyl position of the undeuterated anesthetic molecule. In this method the 1,1 - difluoro - 2 - deutero - 2,2 - dihaloethyl difluoromethyl ether is mixed with heavy water (D20) in the presence of a strong base catalyst at a temperature and for a time sufficient to replace the hydrogen in the 2 - ethyl position of the molecule with deuterium. Similar procedures are described in JACS 83, 1219 (1961) for the preparation of deuterated halothane. The hydrogen - deuterium exchange is an equillibrium reaction, therefore excess heavy water should be present to force the reaction in the direction of the deuterated anesthetic. In general, a ratio of about 10 parts heavy water to about 1 part anesthetic on a weight/weight basis will lead to substantially complete deuteration of the 2 - ethyl position of the molecule. However, any ratio of from 1 to 20 moles D20 per mole anesthetic may be used.
[0006] The strong base catalyst is generally a soluble hydroxide or alkoxide of an alkali metal such as sodium or potassium. Alternatively, a strong base ion exchange resin may be used to catalyze the reaction. The reaction mixture is allowed to react at a temperature of from about 25 to 150°C, with from about 50 to 100°C being preferred. In general, the higher the reaction temperature the more quickly the exchange is completed. For relatively low boiling anesthetics such as enflurane (about 55°C) correspondingly longer reaction times are required. To shorten the reaction time a pressurized reaction vessel may be employed to allow higher reaction temperatures. Phase transfer catalysis may also be used to increase the speed at which the reaction occurs.
EXAMPLE 1
Preparation of Monodeuterated Enflurane
[0008] A 500 ml three-necked flask fitted with a reflux condenser and magnetic stirrer was charged with 100 ml of heavy water (D20) having 99.7% deuterium replacing the hydrogen, 5 grams of anhydrous sodium hydroxide, and 145 grams of enflurane. The mixture was heated at reflux (about 55°C) for about 3 days. The reaction mixture was allowed to cool to room temperature. The ether was separated and dried over calcium chloride. The dry ether was distilled through a four-inch (10 cm.) Vigreaux column, and the fraction boiling at 56-57°C was collected. NMR analysis confirmed this fraction as 90% deuterated enflurane.
EXAMPLE 2
Preparation of 1,1 - Difluoro - 2 - Deutero -
[0009] 2,2 - Dichloroethyl Difluoromethyl Ether A reaction vessel similar to that used in Example 1 above was charged with 200 ml of heavy water, 10 grams of anhydrous sodium hydroxide, and 200 grams of 1,1 - difluoro - 2,2 - dichloroethyl difluoromethyl ether. The reaction mixture was refluxed at about 76°C for about 1.5 hours. Bromine was added dropwise to the crude 1,1 - difluoro - 2 - deutero - 2,2 - dichloroethyl difluoromethyl ether until the red bromine color persisted for several minutes. The resulting mixture was irradiated with a 275 watt sunlamp during bromine addition. The mixture was washed with dilute sodium hydroxide to remove the residual bromine, dried and distilled. The fraction boiling at 87°C was collected. NMR analysis showed this fraction to be 93% CHF2-0-CF2-CCI,D.
EXAMPLE 3
Preparation of 1,1,2 - Trifluoro - 2 - Bromo - 2 - Deuteroethyl difluoromethyl Ether
[0010] In the same manner as described in Examples 1 and 2 above, the reaction vessel was charged with 200 ml of heavy water, 10 grams of anhydrous sodium hydroxide and 200 grams of 2 - bromo - 1,1,2 - trifluoroethyl difluoromethyl ether. The reaction mixture was heated to reflux (about 67°C) and held at that temperature for about 1.5 hours. The reaction mass was cooled, after which the crude ether was separated and dried over calcium chloride. The dry ether was distilled, and the fraction boiling at about 72-73°C was collected. NMP analysis showed this fraction to be greater than 96 percent 1,1,2 - trifluoro - 2 - bromo - 2 - deuteroethyl difluoromethyl ether.
EXAMPLE 4
[0011] Metabolism studies for the presence of inorganic fluorides following the use of monodeuterated enflurane and enflurane were carried out as follows. Enflurane and monodeuterated enflurance were vaporized by metering the liquid compound at a controlled rate into a temperature regulated vaporization flask held at 150°C. The vapor was swept into the air inlet of a 30-liter glass exposure chamber at a rate of 6 liters/minute. The concentration of the anesthetic in the exposure chamber was monitored by gas-liquid chromatography using direct gas sampling loops.
[0012] Groups of 8 male rats (6 months of age, 250-300 grams) were exposed to room air (controls) and 2.5% volume/volume of enflurane _ and monodeuterated enflurane for a period of 3 hours. After exposure, the animals were removed immediately. All animals were maintained in individual cages for 48 hours after exposure. Urine was collected during each of two 24-hour intervals after exposure. No differences were noted between the anesthetic properties of enflurane and monodeuterated enflurane.
[0013] Urinary volume for each animal was recorded, and the urine samples were assayed for inorganic fluoride.
[0014] A comparison of the amount of totai inorganic fluoride in the urine of the control and test animals is shown in Table I below.
EXAMPLE 5
[0015] Using essentially the same technique as described in Example 4, 1,1,2 - trifluoro - 2 - bromoethyl difluoromethyl ether was compared to its mono-deuterated analogue prepared according to the method of Example 3. The rats were exposed to 1.5 percent volume/volume concentration of the control anesthetic and its deuterated analogue for a period of 3 hours. No differences were noted between the anesthetic properties of 1,1,2 - trifluoro - 2 - bromoethyl difluoromethyl ether and the mono-deuterated analogue.
[0016] Urine volume was recorded, and the urine was assayed for inorganic fluoride. The results are shown in Table I. In addition, after 48 hours, the animals were sacrificed, and the blood was collected. Serum bromide ion concentrations were determined, the results of which are shown in Table II.
EXAMPLE 6
[0017] Using essentially the same method as described in Example 4, 1,1 - difluoro - 2,2 - dichloroethyl difluoromethyl ether and its mono- deuterated analogue were compared. Because of the potency of these anesthetics, the rats were exposed to a concentration of only 0.5 percent volume/volume of the anesthetic and its mono-deuterated analogue. Again, no differences in anesthetic properties were noted between 1,1 - difluoro - 2,2 - dichloroethyl difluoromethyl ether and its mono-deuterated analogue.
[0018] The urine was collected and analyzed for inorganic fluoride concentration. The results are recorded in Table I.
[0019] The data indicate that animals treated with the mono-deuterated 1,1 - difluoro - 2,2 - dihaloethyl difluoromethyl ethers, that are the subjects of the present invention, show significantly lower concentrations of inorganic fluoride in the urine of the treated animals than in the urine of similar animals anesthetized using the undeuterated analogues. Likewise, animals treated with 1,1,2 - trifluoro - 2 - bromo - 2 - deuteroethyl difluoromethyl ether showed lower concentrations of inorganic bromide in the serum than did animals treated with undeuterated anesthetic. The most dramatic differences were seen in the mono- deuterated enflurane and 1,1,2 - trifluoro - 2 - bromo - deuteroethyl difluoromethyl ether where a decrease in organic fluoride of 65 percent and 76 percent, respectively, as compared to the undeuterated anesthetics was observed. Although less dramatic, a significant decrease (29 percent) was also observed for 1,1 - difluoro - 2 - deutero - 2,2 - dichloroethyl difluoromethyl ether. Anesthetic potency coupled with a low release of inorganic fluoride into the blood make this latter compound the preferred embodiment of the invention.
1. A deuterated 1,1 - difluoro - 2,2 - dihaloethyl difluoromethyl ether of the formula
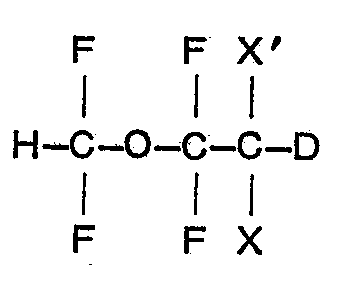
wherein X is fluoro and X' is bromo or chloro, or both X and X' are chloro.
wherein X is fluoro and X' is bromo or chloro, or both X and X' are chloro.
2. Use of a compound claimed in Claim 1 in an anesthetic.
3. A process for preparing a compound claimed in Claim 1 characterised by reacting
an ether of the formula
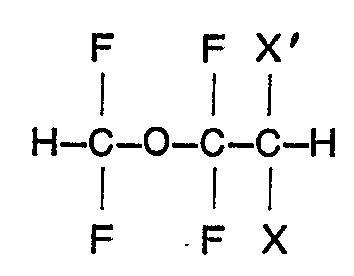
wherein X is fluoro and X' is bromo or chloro,- or both X and X' are chloro, with D20 in presence of a strong base.
wherein X is fluoro and X' is bromo or chloro,- or both X and X' are chloro, with D20 in presence of a strong base.
1. 1,1 - difluoro - 2,2 - dihalo - éthyl - difluorométhyléther deutéré ayant la formule:
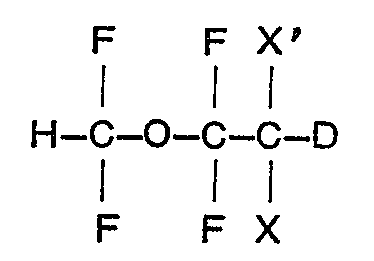
dans laquelle X est du fluor et X' est du brome ou du chlore, ou bien X et XI sont tous les deux du chlore.
dans laquelle X est du fluor et X' est du brome ou du chlore, ou bien X et XI sont tous les deux du chlore.
2. Utilisation du composé selon la revendication 1, dans un anesthésique.
3 Procédé pour préparer un composé selon la revendication 1, caractérisé par le fait
qu'on fait réagir un éther de formule
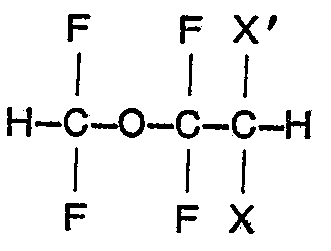
dans laquelle X est du fluor et X' est du brome ou du chlore ou X et X' sont tous les deux du chlore, avec l'eau lourde (D20) en présence d'une base forte.
dans laquelle X est du fluor et X' est du brome ou du chlore ou X et X' sont tous les deux du chlore, avec l'eau lourde (D20) en présence d'une base forte.
1. Deuterierter 1,1 - Difluor - 2,2 - dihalogenethyl - difluor - methylether der Formel
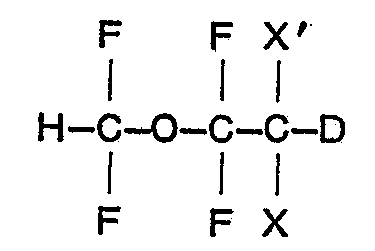
in der X Fluor und X' Brom oder Chlor oder X und X' beide Chlor bedeuten.
in der X Fluor und X' Brom oder Chlor oder X und X' beide Chlor bedeuten.
2. Verwendung einer Verbindung nach Anspruch 1 in einem Anästhetikum.
3. Verfahren zur Hesstellung einer Verbindung nach Anspruch 1, dadurch gekennzeichnet,
daß man einen Ether der Formel
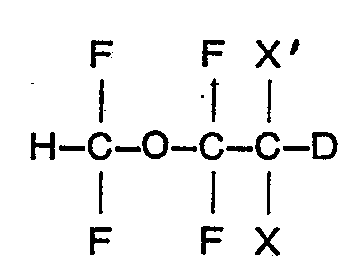
in der X Fluor und X' Brom oder Chlor oder X und X' beide Chlor bedeuten, mit D20 in Gegenwart einer starken Base umsetzt.
in der X Fluor und X' Brom oder Chlor oder X und X' beide Chlor bedeuten, mit D20 in Gegenwart einer starken Base umsetzt.
