|
(11) | EP 0 608 133 A1 |
| (12) | EUROPEAN PATENT APPLICATION |
|
|
|
|
|||||||||||||||||||||||||||
| (54) | A silver halide color photographic material |
| (57) A silver halide color photographic material comprising a support having thereon hydrophilic
colloid layers including a silver halide emulsion layer, wherein at least one of the
hydrophilic colloid layers comprises a cyan dye-forming coupler represented by the
following Formula (I), (II) or (III),
|
FIELD OF THE INVENTION
[0001] This invention relates to a novel cyan coupler for a raw material of a silver halide color photographic light-sensitive material and particularly to a novel cyan couplerfor photographic use capable of forming a dye image excellent in fastness against heat, moisture and light.
BACKGROUND OF THE INVENTION
[0002] Generally in the case of preparing a color photograph, a silver halide color photographic light-sensitive material is exposed to light and is then color-developed. At that time, a dye is produced, in the areas exposed to light, by reacting an oxidized aromatic primary amine color developing agent with a due-forming coupler and a dye-image is formed thereby. In such a photographic process as mentioned above, a color reproduction process is used in a subtractive color system and thereby each of yellow, magenta and cyan color images is formed.
[0003] Heretofore, the above-mentioned couplers for photographic use generally applicable to form a yellow color-image include, for example, an acylacetanilido type coupler, those generally applicable to form a magenta color-image include, for example, a pyrazolone, pyrazolobenzimidazole, pyrazolotriazole or indazolone type coupler, and those generally applicable to form a cyan color-image include, for example, a phenol or naphthol type coupler. The dye-images came out of these couplers have been so required as neither to be discolored not to be faded down, even if the images are exposed to light for a long time or they are stored at a high temperature and a high humidity.
[0004] The researches and practical application of the above-mentioned phenol type and naphthol type couplers have been progressed so far for serving as a coupler for producing a cyan dye. However, they have still not been satisfactory a bit to the points of the spectral absorption characteristics, heat resistance and moisture resistance of a cyan dye-image formed thereby. Therefore, for aiming at the improvements thereof, various proposals, including the selections and investigation of substituents to be put inside a coupler, have so far been made. However, none of such a coupler as satisfiable to every requirement for these characteristics has not yet been discovered.
[0005] Japanese Patent Publication Open to Public Inspection (hereinafter referred to as JP OPI Publication) Nos. 62-278552/1987 and 62-279340/1987 describe each of the pyrroloimidazole type couplers. However, these couplers could not be a cyan coupler, because they have not any electron withdrawing group in the 2nd position and have the absorption of a dye produced thereby in a short wave.
[0006] Taking the situations such as mentioned above into consideration, the present inventors have further progressed their studies and, resultingly, they have discovered a coupler for photographic use that is capable of forming a dye-image having a hue invariable against heat, moisture and light.
SUMMARY OF THE INVENTION
[0007] It is, accordingly, an object of the invention is to provide a novel cyan coupler for photographic use that is applicable to serve as a raw material of a silver halide color photographic light-sensitive material, and another object of the invention is to provide a cyan coupler for color photographic use that is capable of forming a dye-image not producing any variation of the hue thereof caused by heat, moisture and light.
[0008] The above-mentioned objects of the invention can achieved with a coupler for photographic use that is represented by the following Formula (I), (II) or (III).
wherein R , R2, R3 and Y represent each a hydrogen atom or a substituent; EWG represents an electron withdrawing group having a Hammett's substituent constant δP of not less than 0.3 and X represents a hydrogen atom or a group capable of splitting off upon reacting with an oxidized product of a color developing agent, provided that R3 shall not be split off by reacting with an oxidized product of the color developing agent.
[0010] In Formulas (I), (II) and (III), the substituents represented by R , R2, R3 include each of the groups of alkyl, aryl, anilino, acylamino, sulfonamido, alkylthio, arylthio, alkenyl and cycloalkyl. Besides the above, the substituents also include, for example, a halogen atom, each of the groups of cycloalkenyl, alkinyl, heterocyclic, sulfonyl, sulfinyl, phosphonyl, acyl, carbamoyl, sulfamoyl, cyano, alkoxy, aryloxy, heterocyclic-oxy, siloxy, acyloxy, sulfonyloxy, carbamoyloxy, amino, alkylamino, imido, ureido, sulfamoylamino, alkoxycarbonyl amino, aryloxycarbonyl- amino, alkoxycarbonyl, aryloxycarbonyl, heterocyclic-thio, thioureido, carboxy, mercapto, nitro and sulfo, and a spiro compound residual group and a cross-linked hydrocarbon compound residual group.
[0011] Among each of the groups represented by R , R2, R3, the alkyl groups are preferable to have 1 to 32 carbon atoms and may also be straight-chained or branched.
[0013] The acyl amino group include, for example, those of alkylcarbonyl amino and acylcarbonyl amino.
[0015] The alkyl components and aryl components of the alkylthio groups and arylthio groups include an alkyl group and an aryl group each represented by R , R2, R3.
[0016] The alkenyl group is preferable to have 2 to 32 carbon atoms. The cycloalkyl group is preferable to have 3 to 12 carbon atoms and more preferable to have 5 to 7 carbon atoms. The alkenyl group may also be straight-chained or branched.
[0017] The cycloalkenyl group is preferable to have 3 to 12 carbon atoms and more preferable to have 5 to 7 carbon atoms. It is preferable that the sulfonyl groups include alkylsulfonyl and arylsulfonyl; the sulfinyl groups, those of alkylsulfinyl and arylsulfinyl; the phosphonyl groups, those of alkylphosphonyl, alkoxyphosphonyl, aryloxyphosphonyl and arylphosphonyl; the acyl groups, those of alkylcarbonyl and arylcarbonyl; the carbamoyl groups, those of alkylcarbamoyl and arylcarbamoyl, the sulfamoyl groups, those of alkylsulfamoyl and arylsulfamoyl; the acyloxy groups, those of alkylcarbonyloxy and arylcarbonyloxy; the sulfonyloxy groups, those of alkylsulfonyloxy and arylsulfonyloxy; the carbamoyloxy groups, those of alkylcarbamoyloxy and arylcarbamoy- loxy; the ureido groups, those of alkylureido and arylureido; the sulfamoylamino groups, those of alkylsulfamoylamino and arylsulfamoylamino; the heterocyclic groups, those of the 5- to 7-membered including, concretely, those of 2-thienyl, 2-pyrimidinyl, 2-benzothiazolyl, 1-pyrrolyl and 1-tetrazolyl; the heterocyclic-oxy groups include, preferably, those having a 5- to 7-membered heterocyclic ring, such as those of those of 3,4,5,6-tetrahydropyranyI-2-oxy and 1-phenyltetrazole-5-oxy; the heterocyclic-thio groups include, preferably, those having a 5- to 7-membered heterocyclic ring, such as those of 2-pyridylthio, 2-benzothiazolylthio and 2,4-di- phenoxy-1,3,5-triazole-6-thio; the siloxy groups, those of trimethylsiloxy, triethylsiloxy and dimethylbutylsi- loxy; the imido groups, those of succin imido, 3-heptadecyl succin imido, phthalimido and glutarimido; the spiro compound residual groups, those of spiro [3.3] heptane-1-yl; and the cross-linked hydrocarbon compound residual groups, those of bicyclo [2.2.1] heptane-1-yl, tricyclo [3.3.1.13.7] decane-1-yl and 7,7-dimethyl-bicyclo [2.2.1] heptane-1-yl; respectively.
[0018] The above-given groups may also have a further substituent including a ballast group such as a long- chained hydrocarbon group and a polymer residual group.
[0019] In Formulas (I), (II) and (III), the substituents represented by EWG include a substituent having a Ham- mett's substituent constant σP of not less than 0.3. They include typically a cyano group, a nitro group, a sulfonyl group (such as those of octylsulfonyl, phenylsulfonyl, trifluoromethylsulfonyl and pentafluorophenylsulfonyl), a β-carboxyvinyl group, a sulfinyl group (such as those of t-butylsulfinyl, tolylsulfinyl, trifluoromethylsulfinyl and pentafluorophenylsulfinyl), a β, l3-dicyanovinyl group, a halogenated alkyl group (such as those of trifluoromethyl, perfluorooctyl and ω-hydroperfluoro-dodecyl), a formyl group, a carboxyl group, a carbonyl group (such as those of acetyl, pivaloyl, benzoyl and trifluoro-acetyl), an alkyloxycarbonyl or aryloxycarbonyl group (such as those of ethoxycarbonyl and phenoxycarbonyl), 1-tetrazolyl group, 5-chloro-1-tetrazolyl group, a carbamoyl group (such as those of dodecylcarbamoyl and phenylcarbamoyl) and a sulfamoyl group (such as those of trifluoromethylsulfamoyl, phenylsulfamoyl and ethylsulfamoyl).
[0020] The groups represented by X, which are capable of splitting off upon making a reaction thereof with an oxidized product of a color developing agent, include, for example, a halogen atom (such as those of chlorine, bromine and fluorine) and each of the groups of alkylene, alkoxy, aryloxy, heterocyclic-oxy, acyloxy, sulfonyloxy, alkoxycarbonyloxy, aryloxycarbonyl, alkyloxalyloxy, alkoxyoxalyloxy, alkylthio, arylthio, heterocyclic-thio, alkyloxythiocarbonylthio, acylamino, sulfonamido, a nitrogen-containing heterocyclic ring coupled by a N-atom, alkyloxycarbonylamino, aryloxycarbonylamino, and carboxyl. Among them, a hydrogen atom, a halogen atom, an alkoxy group, an aryloxy group, an alkylthio group, an arylthio group and a nitrogen-containing heterocyclic ring group coupled by a N-atom are preferred.
[0021] Y represents a hydrogen atom or a substituent. The preferable substituents include, for example, those capable of splitting off after making a reaction thereof with an oxidized product of a developing agent, which include, for example, a group capable of splitting off under the alkaline conditions, such as those described in JP OPI Publication No. 61-228444/1986 and a substituent capable of coupling off upon making a reaction thereof with an oxidized product of a developing agent such as those described in JP OPI Publication No. 56-133734/1981. Among them, hydrogen atom is preferred to be represented by Y.
[0022] Therefore, among the compounds of the invention represented by Formula (I), (II) or (III), the particularly preferable one is represented by the following Formula (IA), (IIA) or (IIIA), respectively.
[0023] In the formulas, R1, R2, R3, EWG and X are each synonymous with the R1, R2, R3, EWG and X denoted in Formula (I), respectively.
[0024] Now, the representative compounds of the invention will be exemplified below. However, the invention shall not be limited thereto.
[0025] Compounds (I) of the invention can be synthesized in accordance with the synthesizing process described in Journal of the American Chemical Society, Vol.90, No.140, pp.3830-3834, (1968).
Synthesis Example (I)
Synthesis of Intermediate (b)
[0027] (a) of 22.6 g (in 0.2 mols) and a-bromoacetophenone of 19.9 g (in 0.1 mols) are reacted in 200 cc of ethyl acetate at room temperature for 16 hours. After completing the reaction, the resulting crystals are taken out through a filtration and the filtrated crystals are recrystallized with acetonitrile, so that 40 g of intermediate (b) can be obtained (at a yield of 95%).
Synthesis of Intermediate (d)
[0028] Intermediate (b) of 21.3 g (in 0.05 mols) is dispersed in 250 cc of N,N-dimethyl formamide and 10.0 g of potassium carbonate is then added thereto. The resulting mixture is stirred at room temperature for 20 minutes. Then, 11.9 g of(c) (in 0.05 mols) is gradually added and an organic phase is extracted therefrom. The extracted organic phase is dried and condensed. The resulting residue is refined through a column chromatography. Thereafter, the refined residue is recrystallized from methanol, so that 6.10 g of intermediate (d) can be obtained (in a yield of 21%).
Synthesis of Exemplified Compound (12)
[0029] (d) of 5.81 g (in 0.01 mols) is dissolved in 60 cc of ethyl acetate and 0.5 g of 5% palladium carbon is added. They are reacted together at room temperature for 4 hours under one hydrogen atmospheric pressure. After completing the reaction, a catalyst is separated through a filtration and a solvent is distilled off under reduced pressure. The resulting residue is recrystallized from ethanol, so that 3.54 g of the subject exemplified compound (12) can be obtained (in a yield of 72%).
Synthesis Example (II)
Synthesis of Intermediate (c)
[0032] (a) of 18.9 g (in 0.1 mols) (that was synthesized in accordance with the process described in Chemical Heterocyclic Compound, Vo1.11, p.1059, 1975) and (b) of 43.1 g (in 0.1 mols) are dissolved in 200 cc of dimethyl sulfoxide. Then, 11.2 g of potassium-t-butoxide (in 0.1 mols) is gradually added. After a reaction is made at 50°C for 2 hours, the reactant is allowed to cool and is then poured in dilute hydrochloric acid solution, so that an extraction is made with ethyl acetate. The resulting extracted solution is washed with water and a solvent is then distilled off under reduced pressure. Ethyl acetate of 200 cc is added to the resulting residue and the mixture is heatedly reacted for 5 hours under reflux conditions. After completing the reaction, the reactant is washed with water and dried, and the solvent is distilled of under reduced pressure. The resulting residue is recrystallized with ethanol, so that 23.8 g of intermediate (c) (in a yield of 42%) can be obtained.
Synthesis of Exemplified Compound (16)
[0033] Intermediate (c) of 5.66 g (in 0.01 mols) is heated and refluxed, so that a reaction is made for 4 hours. After completing the reaction, the reactant is poured in ice water and neutralized with sodium carbonate. The resulting crystals are taken out through a filtration. The resulting crude crystals are recrystallized with ethanol, so that 3.90 g of the subject exemplified compound (16) (in a yield of 72%) can be obtained.
[0035] Compound (III) of the invention can be synthesized in accordance with the synthesization process described in Arch. Pharm. Vol.325, No.4, pp.225-234, 1992.
Synthesis Example (III)
(i) Synthesis of Intermediate (14c)
[0037] (14a) of 4.40 g (in 0.01 mols) (that was synthesized in the synthesization process described in J. Org. Chem., Vo1.29, p.3459, 1964) and (14b) of 3.24 g (in 0.02 mols) are heated and refluxed in 30 ml of acetone for 3 hours. After completing the reaction, a solvent is distilled off under reduced pressure and an organic layer is extracted by adding ethyl acetate and water. Thereafter, the residue obtained by distilling the solvent under reduced pressure is refined in a column chromatography. Thereafter, a crystallization is further made with ethanol, so that 2.47 g of intermediate (14c) (in a yield of 49%) can be obtained.
(ii) Synthesis of Intermediate (14d)
[0038] Intermediate (14c) of 5.04 g (in 0.01 mols) and zinc of 6.53 g (in 0.1 mols) are dispersed in 50 ml of acetone and 10 ml of conc. sulfuric acid is dropped therein at room temperature. After completing the dropping it, a further reaction is made for one hour at room temperature. After completing the reaction, the resulting insoluble matter is separated through a filtration. Then, ethyl acetate and water are added thereto. After completing the neutralization, an organic layer is extracted out. Thereafter, the residue obtained by distilling off a solvent under reduced pressure is refined in a column chromatography, so that 3.53 g of intermediate (14d) (in a yield of 88%) can be obtained.
(iii) Synthesis of Intermediate (14f)
[0039] Intermediate (14d) of 4.01 g (in 0.01 mols) and (14e) of 4.26 g (in 0.02 mols) are reacted in 20 ml of N,N-dimethyl formamide at 110°C for 3 hours. After completing the reaction, ethyl acetate and water are added thereto, so that an organic layer is extracted. Thereafter, a solvent is distilled off under reduced pressure. The resulting residue is refined in a column chromatography, so that 4.01 g of intermediate (14f) (in a yield of 62%) can be obtained.
(iv) Synthesis of Exemplified Compound (14)
[0040] Intermediate (14f) of 6.47 g (in 0.01 mols) is dissolved in 60 ml of acetic acid and zinc of 1.96 g (in 0.03 mols) is gradually added thereto at room temperature. Then, a reaction is made for 3 hours at room temperature. After completing the reaction, ethyl acetate and water are added and, after completing a neutralization, an organic layer is extracted. Thereafter, a solvent is distilled off under reduced pressure and the resulting residue is recrystallized with ethanol, so that 3.97 g of the subject exemplified compound (14) (in a yield of 77%) can be obtained.
[0042] The couplers of the invention may be ordinarily used each in an amount within the range of 1x1 0-3 to 1 mol and, preferably, 1x10-2 to 8x10-1 mols per mol of silver halide.
[0043] The couplers of the invention may be used with other kinds of cyan couplers in combination. To the couplers of the invention, the processes and techniques used in any common dye-forming couplers may be similarly applied.
[0044] The couplers of the invention may be used as a raw material for forming a color photograph obtained in any color development processes including, concretely, a coupler-in-developer type color development process and a coupler-in-emulsion type color development process. When the couplers of the invention are used in a coupler-in-developer type color development process, the couplers of the invention can be used by dissolving them in an aqueous alkaline solution or an organic solvent (such as alcohol) and the resulting solution is then added in a development processing solution.
[0045] When the couplers of the invention are used as a raw material for forming a color photograph in a coupler-in-emulsion type color development process, the couplers of the invention are used by containing them in a photographic light-sensitive material. Typically, the following process can preferably be used; the couplers of the invention are compounded in a silver halide emulsion and the emulsion is coated on a support, so that a color light-sensitive material can be formed.
[0046] The couplers of the invention are used in such a color photographic light-sensitive material as a color negative or positive film and a color printing paper.
[0047] The above-mentioned light-sensitive materials including color printing paper, in which the couplers of the invention are used, may be for the monochromatic and multicolor use. In a multicolor type light-sensitive material, the couplers of the invention may be contained in any layers. It is, however, usual to contain them in a red light-sensitive silver halide. Amulticolortype light-sensitive material has the dye-image forming component units sensitive to the three primary-color regions of spectra, respectively. Each of the component units may be comprised of a single or multilayered emulsion layer having a sensitivity to a specific region of spectra. The component layers of a light-sensitive material, including the layers as the image-forming component units, may be arranged in a variety of orders as well known in the art.
[0048] Atypical multicolor type light-sensitive material is comprised of a support bearing thereon a cyan dye-image forming component unit comprising at least one red light-sensitive silver halide emulsion layer containing at least one cyan coupler (in which at least one cyan coupler is a cyan coupler of the invention), a magenta dye-image forming component unit comprising at least one green light-sensitive silver halide emulsion layer containing at least one magenta coupler and a yellow dye-image forming component unit comprising at least one blue light-sensitive silver halide emulsion layer containing at least one yellow coupler.
[0049] Alight-sensitive material may have an additional layer such as a filter layer, an interlayer, a protective layer and an undercoat layer.
[0050] A coupler of the invention may be contained in an emulsion in a conventionally known method. For example, after a coupler of the invention is dissolved independently or combination in a high boiling organic solvent having a boiling point of not lower than 175°C such as tricresyl phosphate and dibutyl phthalate or a low boiling solvent such as butyl propionate independently or, if required, a mixed solution thereof, the resulting solution is mixed with an aqueous gelatin solution containing a surfactant and the resulting mixture is emulsified by a high-speed rotary mixer or a colloid mill. Then, a silver halide is added thereto, so that a silver halide emulsion applicable to the invention can be prepared.
[0051] The silver halide compositions preferably applicable to a light-sensitive material containing a coupler of the invention include, for example, silver chloride, silver chlorobromide or silver chloroiodobromide and, further, a mixture thereof such as a mixture of silver chloride and silver bromide may also be applied thereto. To be more concrete, when a silver halide emulsion is applied to a color printing paper, a rapid developability is particularly required. It is, therefore, preferable to contain a chlorine atom as a halogen composition of a silver halide. It is particularly preferable to contain silver chloride, silver chlorobromide or silver chloroiodobromide each containing at least 1% of silver chloride.
[0052] A silver halide emulsion is chemically sensitized in an ordinary method and can also be spectrally sensitized to any desired wavelength region.
[0053] For the purposes of preventing a fog production and/orkeeping the stability of photographic characteristics in the courses of preparing, preserving or photographic processing a light-sensitive material, a compound having been known in the art as an antifoggant or a stabilizer can be added to a silver halide emulsion.
[0054] An anticolor-foggant, a dye-image stabilizer, a UV absorbent, an antistatic agent, a matting agent a surfactant and so forth, which are commonly used in a light-sensitive material, may be used in a color light-sensitive material containing a coupler of the invention.
[0055] The above-mentioned additives may be referred to, for example, Research Disclosure, Voi.176, pp.22-31, (Dec., a978).
[0056] A color photographic light-sensitive material containing a coupler of the invention is capable of forming an image in any color development processes having been known in the art.
[0057] A color photographic light-sensitive material using a coupler relating to the invention and containing a color developing agent as either itself or its precursor in a hydrophilic colloidal layer thereof may also be processed in an alkali-activated bath.
[0058] After color-developing a color photographic light-sensitive material using a coupler of the invention, it is then bleached and fixed. The bleaching and fixing treatments may also be carried out at the same time.
[0059] After completing the fixing treatment, a washing treatment is usually carried out. It is also allowed to carry out a stabilizing treatment in place of a washing treatment or the both treatments may further be carried out in combination.
EXAMPLES
[0060] Now, the invention will concretely be detailed with reference to the following examples. However, the invention shall not be limited thereto.
Example 1
[0061] Red-sensitive color photographic light-sensitive material sample 1 was prepared by coating the following layers on a paper support laminated with polyethylene on the both sides thereof, in the order from the support side. The amounts of the compounds added will be indicated in terms per sq.meter unless otherwise expressly stated, (provided that the amounts of silver halides used will be indicated in terms of a silver content thereof.)
Layer 1: An emulsion layer
[0062] This layer was comprised of a comparative cyan coupler (a) in an amount of 9.1x1 0-4 mols dissolved with 1.3 g of gelatin, 0.21 g of a red-sensitive silver chlorobromide emulsion (containing silver chloride of 99.5 mol%) and 0.45 g of dioctyl phosphate.
Layer 2: A protective layer
[0063] This layer was a protective layer containing 0.50 g of gelatin. To this layer, sodium 2,4-dichloro-6-hydroxy- s-triazine was also added so as to be in an amount of 0.017 g per g of gelatin.
[0064] Next, Samples 2 through 24 were each prepared in quite the same manner as in Sample 1, except that comparative coupler (a-1) was replaced by a coupler shown in Table 1 in the same amount as that of the comparative coupler (a).
[0065] The resulting Samples 1 through 8 were each exposed to light through a wedge in an ordinary method and were then developed in the following steps.
Color developing solution
[0066] With the processed Samples 1 through 8, the densities thereof were measured through a densitometer (Model KD-7 manufactured by Konica Corp.). Each of the processed samples was then allowed to stand for 14 days under the atmospheric conditions of a high temperature and a high humidity (at 60°C and 80%RH) and the heat resistance and moisture resistance of the resulting dye-images were checked up.
[0067] Each of the samples was exposed to Xenon rays emitted from a Xenon fade-o-meter for 10 days and the resulting density thereof was measured to check up the light fastness. The results thereof will be shown in Table 1; provided, therein the heat resistance, moisture resistance and light fastness of each dye-images will be indicated by a dye residual percentage obtained after testing the heat resistance, moisture resistance and light fastness at an initial density of 1.0.
[0068] As is obvious from the results shown in Table 1, it was proved that every sample applied with the couplers of the invention was high in dye residual percentage, excellent in heat and moisture resistance and fast against light, as compared to the samples applied with the comparative couplers.
Example 2
[0069] A red-sensitive color light-sensitive material (Sample 23) was prepared by coating the following layers on a subbed triacetate film in the order from the support side. The amounts of the additives added thereto are indicated by an amount per sq.meter, unless otherwise expressly stated, (provided that the amounts of silver halides used therein are indicated by an amount in terms of the silver contents.
Layer 1: An emulsion layer
[0070] This layer was a red-sensitive emulsion layer comprising comparative cyan coupler (b) in an amount of 8.0x10-4 mols that was dissolved with 1.4 g of gelatin, 1.5 g of a red-sensitive silver iodobromide emulsion (containing 4 mol% of silver iodide) and 1.1 g of tricresyl phosphate.
Layer 2: A protective layer
[0071] This layer was a protective layer containing 1.5 g of gelatin. Sodium 2,4-dichloro-6-hydroxy-s-triazine was also added thereto as a layer hardener so that the contents thereof could be 0.017 g per g of gelatin.
[0072] Samples 24 through 44 were prepared in quite the same manner as in Sample 23, except that Comparative Coupler b-1 was replaced by the couplers shown in Table 2, (provided, the couplers were each added in the same mols as that of Comparative coupler b-1).
[0073] The resulting film samples were exposed to light through a wedge in an ordinary method and were then color-developed in the following color processing steps.
The compositions of the processing solutions used in the processing steps were as follows.
[0075] With the processed Samples 23 through 44, the densities thereof were measured through a densitometer (Model KD-7 manufactured by Konica Corp.). Each of the processed samples was then allowed to stand for 14 days under the atmospheric conditions of a high temperature and a high humidity (at 60°C and 80%RH) and the heat resistance and moisture resistance of the resulting dye-images were checked up.
[0076] Each of the samples was exposed to Xenon rays emitted from a Xenon fade-o-meter for 10 days and the resulting density thereof was measured to check up the light fastness. The results thereof will be shown in Table 2; provided, therein the heat resistance, moisture resistance and light fastness of each dye-images will be indicated by a dye residual percentage obtained after testing the heat resistance, moisture resistance and light fastness at an initial density of 1.0.
[0077] The color-image produced on each sample was enlarged 10 times as large as the original on a Konica color paper, and a color paper development process (in CPK-18P) was carried out. The resulting color reproducibility was evaluated by 5 grades with the eye. The evaluation is indicated that the higher the evaluation value was, the more the color reproducibility was excellent.
[0078] As is obvious from the results shown in Table 2, it was proved that every sample applied with the couplers of the invention was high in dye residual percentage, excellent in heat-moisture resistance, fast against light and also excellent in color reproducibility, as compared to the samples applied with the comparative coupler b-1. It was also proved that the samples applied with the couplers of the invention were excellent in reproducibility, as compared to the samples applied with comparative coupler c-1, c-2 or c-3.
Example 3
[0079] Samples 45 through 60 of the red-sensitive color reversal type photographic light-sensitive materials containing the couplers shown in Table 3 were each prepared respectively by coating the following layers on a triacetyl cellulose film support in the order from the support side.
Layer 1: An emulsion layer
[0080] This layer was a red-sensitive emulsion layer comprising the couplers shown in Table 3 in an amount of 9.1x10-4 mols each dissolved with 1.4 g of gelatin, 0.5 g of a red-sensitive silver chlorobromide emulsion (containing 96 mol% of silver chloride) and 1.5 g of dibutyl phthalate.
Layer 2: A protective layer
[0081] This layer was a protective layer containing 0.5 g of gelatin. To this layer, sodium 2,4-dichloro-6-hydroxy- s-triazine was also added so as to be in an amount of 0.017 g per g of gelatin.
[0082] The resulting Samples 1 through 8 were each exposed to light through a wedge in an ordinary method and were then developed in the following steps.
[0085] With each of the processed samples, the heat-moisture resistance and light fastness of the resulting dye-images were checked up in the same manner as in Example 2. The results thereof will be shown in Table 3.
[0086] As is obvious from the results shown in Table 3, it was proved that every sample applied with the couplers of the invention was high in dye residual percentage, excellent in heat and moisture resistance and fast against light, as compared to the samples applied with the comparative couplers.
Example 4
[0087] On a transparent polyethylene terephthalate film support, a heat-development type light-sensitive layer comprising the following components in the amounts indicated below, so that a heat-development type light-sensitive material was thereby prepared.
Silver iodobromide (converted into Ag content)
[0089] After the resulting light-sensitive material was exposed imagewise to light, it was overlapped with an image-receiving material prepared by coating polyvinyl chloride on a photographic baryta paper. When heat- developing it at 150°C for 1 minute, an cyan-colored transferred image could be obtained on the image-receiving material.
1. A silver halide color photographic material comprising a support having thereon
hydrophilic colloid layers including a silver halide emulsion layer, wherein at least
one of the hydrophilic colloid layers comprises a cyan dye-forming coupler represented
by the following Formula (I), (II) or (III),
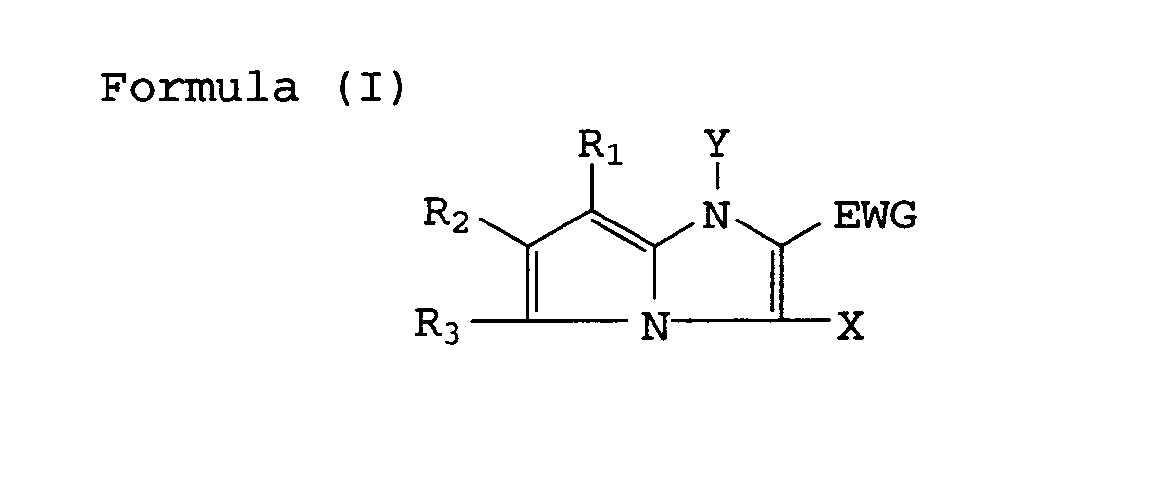
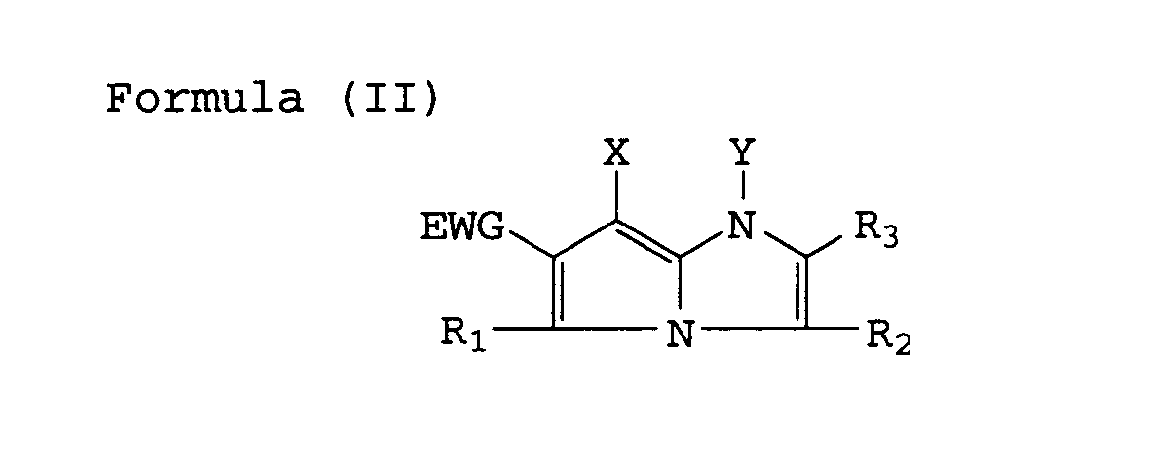
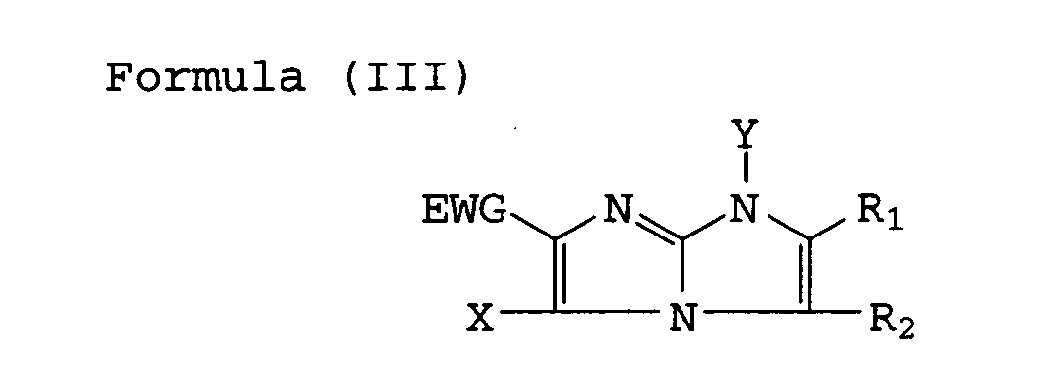
wherein R , R2, R3 and Y each represent a hydrogen atom or a substituent; EWG represents an electron-withdrawing group having a Hammet's substituent constant σP of not less than 0.3; and X represents a hydrogen atom or a group capable of splitting off upon reaction with an oxidized product of a color developing agent.
wherein R , R2, R3 and Y each represent a hydrogen atom or a substituent; EWG represents an electron-withdrawing group having a Hammet's substituent constant σP of not less than 0.3; and X represents a hydrogen atom or a group capable of splitting off upon reaction with an oxidized product of a color developing agent.
2. The color photographic material of claim 1, wherein EWG is a group selected from
a cyan group, a nitro group, a sulfonyl group, β-carboxyvinyl group, a sulfinyl group,
β, β-dicyanovinyl group, a halogenoalkyl group, a formyl group, a carboxyl group,
a carbonyl group, an alkyloxycarbonyl group, an aryloxycarbonyl group, 1-tetrazoryl
group, 5-chloro-1-tetrazolyl group, a carbamoyl group and a sulfamoyl group.
3. The color photographic material of claim 1, wherein Y is a hydrogen atom.
4. The color photographic material of claim 1, wherein said coupler is contained in
an amount of 1x10-3 to 1 mol per mol of silver halide.
5. The color photographic material of claim 1, wherein at least one of the hydrophilic
colloid layers is a silver halide emulsion layer comprising chloride-containing silver
halide grains.
6. A cyan coupler of formula (I) as defined in claim 1.
7. A cyan coupler of formula (II) as defined in claim 1.
8. A cyan coupler of formula (III) as defined in claim 1.
