|
(11) | EP 0 608 149 A1 |
| (12) | EUROPEAN PATENT APPLICATION |
|
|
|
|
|||||||||||||||||||||||||||
| (54) | Fuel additives |
| (57) An ester is used as an additive in a liquid hydrocarbon compression-ignition fuel
oil for reducing consumption of the fuel oil operated in a compression-ignition engine. |
[0001] This invention relates to additives for reducing fuel consumption in compression-ignition (or diesel) engines.
[0002] The art describes esters as additives for diesel engine fuel. For example, US-A-2,527,889 describes polyhydroxy alcohol esters as primary anti-corrosion additives in diesel engine fuel, and GB-A-1,505,302 describes ester combinations including, for example, glycerol monoesters and glycerol diesters as diesel fuel additives, the combinations being described as leading to advantages including less wear of the fuel-injection equipment, piston rings and cylinder liners. However, neither document describes use ofthe esters for reducing fuel consumption.
[0003] EP-A-0227 218 describes a method of reducing fuel consumption in an automotive internal combustion engine which comprises operating said engine on a gasoline hydrocarbon fuel, especially an unleaded fuel, containing an effective fuel reducing amount of a selected additive which is an hydroxyl-containing ester of a monocarboxylic acid and a glycol ortrihydric alcohol, said esteradditive having at least one free hydroxyl group.
[0004] In accordance with the present invention, it has been found that use of certain esters in compression-ignition (or diesel) engines surprisingly reduces fuel consumption. EP-A-0227 218 neither describes nor suggests such use. Also, gasoline and diesel engines have very different characteristics such as different fuel, compression ratios, ignition systems, ignition processes, air/fuel ratios, methods of mixing air with fuel, and injection pressures. Those skilled in the art would not therefore expect a use or effect in one engine to be applicable in the other engine.
[0005] A first aspect of the invention is the use of an ester as an additive in a liquid hydrocarbon compression-ignition fuel oil for reducing consumption of the fuel oil operated in a compression-ignition engine, the ester being an ester of a carboxylic acid and an alcohol wherein the acid has from 2 to 50 carbon atoms and the alcohol has one or more carbon atoms.
[0006] A second aspect of the invention is a method of reducing fuel consumption of a compression-ignition engine operated on a liquid hydrocarbon compression-ignition fuel oil, which method comprises operating the engine on a liquid hydrocarbon compression-ignition fuel oil containing , as an additive, a minor proportion of an ester as defined in the first aspect, sufficient to reduce said fuel consumption.
[0007] The examples of this specification will demonstrate the efficacy of the additives of the invention in reducing fuel consumption.
[0009] The acid from which the ester is derived may be a mono or polycarboxylic acid such as aliphatic, saturated or unsaturated, straight or branched chain, mono and dicarboxylic acids being preferred. For example, the acid may be generalised in the formula
where x represents an integer and is 1 or more such as 1 to 4, and R' represents a hydrocarbyl group having from 2 to 50 carbon atoms and which is mono or polyvalent corresponding to the value of x, the - COOH groups, when more than one is present, optionally being substituent on different carbon atoms from one another.
[0010] 'Hydrocarbyl' means a group containing carbon and hydrogen which group is connected to the rest of the molecule via a carbon atom. It may be straight or branched chain which chain may be interrupted by one or more hetero atoms such as O, S, N or P, may be saturated or unsaturated, may be aliphatic or alicyclic or aromatic including heterocyclic, or may be substituted or unsubstituted. Preferably, when the acid is monocarboxylic, the hydrocarbyl group is an alkyl group or an alkenyl group having 10 (e.g. 12) to 30 carbon atoms, i.e. the acid is saturated or unsaturated. The alkenyl group may have one or more double bonds, such as 1, 2 or 3. Examples of saturated carboxylic acids are those with 10 to 22 carbon atoms such as capric, lauric, myristic, palmitic, and behenic acids and examples of unsaturated carboxylic acids are those with 10 to 22 carbon atoms such as oleic, elaidic, palmitoleic, petroselic, riconoleic, eleostearic, linoleic, linolenic, eicosanoic, galoleic, erucic and hypogeic acids. When the acid is polycarboxylic, having for example from 2 to 4 carboxy groups, the hydrocarbyl group is preferably a substituted or unsubstituted polymethylene.
(ii) Alcohol
[0011] The alcohol from which the ester is derived may be a mono or polyhydroxy alcohol such as a trihydroxy alcohol. For example, the alcohol may be generalised in the formula
where y represents an integer and is 1 or more and R2 represents a hydrocarbyl group having 1 or more carbon atoms such as up to 10 carbon atoms, and which is mono or polyvalent corresponding to the value of y the -OH groups, when more than one is present, optionally being substituent on different carbon atoms from one another.
[0012] 'Hydrocarbyl' has the same meaning as given above for the acid. For the alcohol, the hydrocarbyl group is preferably an alkyl group or a substituted or unsubstituted polymethylene group. Examples of monohydric alcohols are lower alkyl alcohols having from 1 to 6 carbon atoms such as methyl, ethyl, propyl and butyl alcohols.
[0013] Examples of polyhydric alcohols are aliphatic, saturated or unsaturated, straight chain or branched alcohols having 2 to 10, preferably 2 to 6, more preferably 2 to 4, hydroxy groups, and having 2 to 90, preferably 2 to 30, more preferably 2 to 12, most preferably 2 to 5, carbon atoms in the molecule. As more particular examples the polyhydric alcohol may be a glycol or diol, or a trihydric alcohol such as glycerol. (iii) The Esters
[0015] Examples of esters that may be used are lower alkyl esters, such as methyl esters, of the above exemplified saturated or unsaturated monocarboxylic acids. Such esters may, for example, be obtained by saponification and esterification of natural fats and oils of plant or animal origin or by their transesterification with lower aliphatic alcohols.
[0016] Examples of esters of polyhydric alcohols that may be used are those where all of the hydroxy groups are esterified, those where not all of the hydroxy groups are esterified, and mixtures thereof. Specific examples are esters prepared from trihydric alcohols are one or more of the above-mentioned saturated or unsaturated carboxylic acids, such as glycerol monoesters and glycerol diesters, e.g. glycerol monooleate, glycerol dioleate and glycerol monostearate. Such polyhydric esters may be prepared by esterification as described in the art and/or may be commercially available.
FUEL OIL
[0017] This invention is preferably applicable to middle distillate fuel oils which generally boil within the range of about 100°C to about 500°C, e.g. about 150°C to about400°C. The fuel oil can comprise atmospheric distillate or vacuum distillate, or cracked gas oil or a blend in any proportion of straight run and thermally and/or catalytically cracked distillates, or may be a vegetable oi I or a derivative thereof such as an ester made by saponification and re-esterification or by transesterification. The most common petroleum distillates are kerosene, jet fuels, diesel fuels, heating oils and heavy fuel oils. The heating oil may be a straight atmospheric distillate, or it may contain amounts, e.g. up to 35% by weight of vacuum gas oil or of cracked gas oils or of both.
[0018] The concentration of the additive of the invention in the fuel oil may be up to 250,000 ppm such as 1 to 2000 ppm (active ingredient) by weight perweight of fuel, preferably 5 or 10 to 500 or 1000 ppm, more preferably 5 or 10 to 200 or 500 ppm.
[0019] The additive may be incorporated into bulk fuel oil by methods known in the art. Conveniently, the additive may be so incorporated in the form of a concentrate comprising an admixture of the additive and a liquid carrier medium compatible with the fuel oil, the additive being dispersed in the liquid medium. Such concentrates preferably contain from 3 to 75 wt%, more preferably 3 to 60 wt%, most preferably 10 to 50 wt% of the additive, preferably in solution in the oil. Examples of carrier liquid are organic solvents including normal paraffins, isoparaffins, naphthenics, aromatics and alcohols. The carrier liquid must of course be selected having regard to its compatibility with the additive and with the fuel.
[0020] Preferably, the fuel oil has one or more of a sulphur concentration of 0.2% by weight or less.
[0021] More preferably, the sulphur concentration is 0.05% by weight or less, such as 0.01% by weight or less. The art describes methods for reducing the sulphur concentration of hydrocarbon distillate fuels, such methods including for example solvent extraction, sulphuric acid treatment, and hydrodesulphurisation.
CO-ADDITIVES
[0022] The additives of the invention may be used singly or as mixtures of more than one additive. They may also be used in combination with one or more co-additives such as known in the art, for example the following: detergents, antioxidants (to avoid fuel degradation), corrosion inhibitors, dehazers, demulsifiers, metal deactivators, antifoaming agents, cetane improvers, cosolvents, package compatibilisers, lubricity additives, and middle distillate cold flow improvers.
EXAMPLES
Example 1
[0024] The following materials, components and procedures were used and the results were as follows.
Additive
[0025] A: An ester obtained by reacting a C36 dimer acid with ethylene glycol and 'neutralising' acid groups with methanol.
B: Glycerol monoleate
Fuel
Engine
PSAXUD9 1.9L, 4 Cylinder in line
Indirect Injection Diesel Engine
Tests
[0027] The following were done sequentially:
The engine was run for two hours on untreated base fuel at 4000 rpm and 75% load; the engine was then run on fuel treated with the additive for two hours:
The fuel consumption was measured at intervals of 10 minutes throughout the whole period of the testing.
Results
1. The use of an ester as an additive in a liquid hydrocarbon compression-ignition
fuel oil for reducing consumption of the fuel oil operated in a compression-ignition
engine, the ester being an ester of a carboxylic acid and an alcohol wherein the acid
has from 2 to 50 carbon atoms and the alcohol has one or more carbon atoms.
2. The use of claim 1 wherein the acid from which the ester is derived has the general
formula
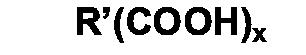
where x represents an integer from 1 to 4 and R' represents a hydrocarbyl group.
where x represents an integer from 1 to 4 and R' represents a hydrocarbyl group.
3. The use of claim 2 wherein the acid is a monocarboxylic acid having 10 to 30 carbon
atoms, which acid is saturated or is unsaturated by having 1 to 3 double bonds in
an alkenyl group.
4. The use of claim 3 wherein the acid has 12 to 22 carbon atoms.
5. The use of claim 4 wherein the acid is a polycarboxylic acid having from 2 to 4
carboxy groups and R' is a substituted or unsubstituted polymethylene group.
6. The use of claim 5 wherein the acid is a dicarboxylic acid.
7. The use of any of the preceding claims wherein the alcohol is an alkyl alcohol
having from 1 to 6 carbon atoms.
8. The use of claim 7 wherein the alcohol is methanol.
9. The use of any of the preceding claims wherein the alcohol is an aliphatic, saturated
or unsaturated, straight chain or branched polyhydric alcohol having 2 to 10 hydroxy
groups and 2 to 90 carbon atoms in the molecule.
10. The use of claim 9 wherein the alcohol has 2 to 4 hydroxy groups and 2 to 5 carbon
atoms in the molecule.
11. The use of claim 10 wherein the alcohol is glycerol.
12. The use of claim 11 wherein the ester is glycerol monooleate.
13. The use of any of the preceding claims wherein the concentration of additive in
the fuel oil is in the range of 1 to 2,000 ppm by weight of active ingredient per
weight of fuel oil.
14. The use of claim 13 wherein the concentration is 5 to 500 ppm.
15. The use of any of the preceding claims wherein the fuel oil has a sulphur concentration
of 0.2% by weight or less based on the weight of the fuel.
16. The composition, use or method of any of claims 1 to 4 wherein the sulphur concentration
is 0.05% by weight or less.
17. The composition, use or method of claim 5 wherein the sulphur concentration is
0.01% by weight or less.
18. A method of reducing fuel consumption of a compression-ignition engine operated
on a liquid hydrocarbon compression-ignition fuel oil, which method comprises operating
the engine on a liquid hydrocarbon compression-ignition fuel oil containing , as an
additive, a minor proportion of an ester as defined in claim 1, sufficient to reduce
said fuel consumption.
