|
(11) | EP 3 663 422 A1 |
| (12) | EUROPEAN PATENT APPLICATION |
| published in accordance with Art. 153(4) EPC |
|
|
|
|
|||||||||||||||||||||||||||
| (54) | CORROSION-RESISTANT ALLOY |
| (57) The invention relates to metallurgy and more particularly to nickel-based alloys
intended for use in aggressive oxidising environments. The present nickel-based corrosion-resistant
alloy contains: ≤ 0.006 wt.% carbon, ≤ 0.1 wt.% silicon, ≤ 1.0 wt.% manganese,
22.8-24.0 wt.% chromium, ≤ 0.75 wt.% iron, 12.0-14.0 wt.% molybdenum, 0.01-0.03
wt.% niobium, 0.01-0.06 wt.% titanium, 0.1-0.2 wt.% aluminium, 0.005-0.01 wt.% magnesium,
≤ 0.015 wt.% phosphorus and < 0.012 wt.% sulphur, with the remainder being nickel
and unavoidable impurities. |
[0001] The invention relates to metallurgic engineering, to nickel-based alloys intended for use in aggressive oxidizing environments.
[0002] A corrosion-resistant alloy Nicrofer 6616 hMo alloy C-4 (No. 2.4610), containing wt.%: 14.5-17.5 Cr, 14.0-17.0 Mo, ≤3.0 Fe, ≤0.009 C, ≤1.0 Mn, ≤0.05 Si, ≤2.0 Co, ≤0.7 Ti, ≤0.020 P, ≤0.010 S, nickel and other unavoidable impurities is known from the prior art (Catalogue "Corrosion-resistant, heat-resistant and high-strength steels and alloys", M., Prometey-Splav, 2008, pp. 304 - 306).
[0003] The alloy is used for the manufacture of equipment operated in a wide range of chemical environments, at room and elevated temperatures. In particular, for adsorbers in flue gas desul-phuring; etching baths and acid recovery plants; acetic acid and agrochemicals plants.
[0004] The nearest analogue of the given invention is an alloy XH65MB
(
Π760) containing, wt.%: ≤0.02 C, ≤0.1 Si, ≤1.0 Mn, 14.5-16.5 Cr, 15.0-17.0 Mo, 3.0-4.5 W, ≤0.5 Fe, ≤0.012 S, ≤0.015 P, nickel and other unavoidable impurities (GOST 5632-2014 - prototype).
[0005] The alloy is used for the manufacture of welded structures (columns, heat exchangers, reactors) operating under elevated temperatures in aggressive redox environments, in the chemical, petrochemical industry (production of acetic acid, epoxy resins, vinyl acetate, melamine, complex organic compounds) and other industries in the temperature range -70 to 500°C.
[0006] The XH65MB alloy and its welded joints can be used in KCl - AlCl3 - ZrCl4 media only up to 500 °C, because at a temperature above this value, the alloy, in addition to intergranular corrosion and corrosion cracking, sharply decreases the percentage elongation from 48% to 7.3-13% at 550°C and up to 2.5% at 625°C and the embrittlement of the metal appears when deformation is applied.
[0007] The objective of the invention is to create an alloy having a high level of corrosion properties at temperatures up to T = 650°C in the working media of chloride plants (KCl - AlCl3 - ZrCl4).
[0008] The technical result of the invention is to obtain an alloy with a high level of plastic properties for the operation in the temperature range 550°C to 625°C and increased corrosion cracking resistance in chlorides KCl, AlCl3 + (ZrCl4 HfCl4) molten metal, at temperatures up to 650°C.
[0009] The specified technical result is achieved in that the alloy containing carbon, silicon, manganese, chromium, molybdenum, phosphorus, sulphur, iron, nickel and unavoidable impurities, according to the invention additionally contains titanium, aluminium, niobium, magnesium with the following components ratio, wt.% :
| Carbon | ≤0.006 |
| Silicon | ≤0.1 |
| Manganese | ≤1.0 |
| Chromium | 22.8-24.0 |
| Iron | ≤0.75 |
| Molybdenum | 12.0-14.0 |
| Niobium | 0.01-0.03 |
| Titanium | 0.01-0.06 |
| Aluminium | 0.1-0.2 |
| Magnesium | 0.005-0.01 |
| Phosphorus | ≤0.015 |
| Sulphur | ≤0.012 |
| Nickel and unavoidable impurities | balance |
[0010] To obtain a stable structure and plastic properties, it is preferable that the content of chromium, molybdenum and iron is related by the ratio:
(the ratio of the total weight percentage of chromium and molybdenum to the percentage of iron is not less than 46.4)
[0011] To obtain a stable structure and high corrosion properties, it is preferable that the content of niobium and carbon is related by the ratio:
(the ratio of the weight percentage of niobium to the weight percentage of carbon is not less than 1.66).
[0012] It is preferably that the content of chromium, molybdenum, iron, niobium and carbon is related by the ratios:
[0014] Comparative analysis with the prototype allows making a conclusion that the claimed alloy differs from the known one with a lower carbon content (≤0.006% instead of ≤0.02), molybdenum (12.0-14.0% instead of 15.0-17.0%), increased chromium content (23.0-24.0% instead of 14.5-16.5%), iron (≤0.75% instead of ≤0.5%) does not contain tungsten, as well as with the additional introduction of elements such as niobium in an amount of 0.01-0.03%, titanium in an amount of 0.01-0.06%, aluminium in an amount of 0.1-0.2% and magnesium in an amount of 0.005-0.01%.
[0015] Moreover, in particular cases of the invention, the claimed ratios of elements are observed:
or
[0017] The limits of the content of alloying elements in the invention alloy are specified as a result of a study of alloys properties with different composition options.
[0018] Exceeding the carbon content of more than 0.006% leads to a decrease in corrosion resistance in solutions of zirconium and hafnium salts due to an increase in the carbide formation process at high temperatures (the appearance of undesirable carbide phases).
[0019] The chromium content was found to be 22.8 - 24.0% to ensure the required heat resistance in hafnium and zirconium oxides. When chromium is introduced into the alloy in the amount of less than 22.8%, the required heat resistance is not ensured, and exceeding the content above 24.0% impairs the heat resistance of the alloy.
[0020] The introduction of molybdenum into nickel alloys increases the recrystallization temperature of solid solutions, inhibits their softening, increases heat resistance, and leads to an ductility increase during short and long tests.
[0021] The range of molybdenum content of 12.0-14.0% is selected to provide the required mechanical properties for both short-term and long-term loads and high temperatures. With the introduction of less than 12.0% of molybdenum, the mechanical properties are not met. When the content is above 14.0%, there is a decrease in ductility and, accordingly, a decrease in the processability of the alloy during metallurgical processing.
[0022] Niobium in an amount of 0.01-0.03%, binds residual carbon and nitrogen to carbides, nitrides and carbonitrides, prevents the formation of chromium carbides and carbonitrides along the grain boundaries. The addition of niobium in an amount 6 to 10 times higher than the carbon content in the alloy eliminates intergranular corrosion of the alloys and protects the welds from destruction. When the niobium content is less than 0.01%, its interaction with residual carbon is ineffective, and the niobium content above 0.03% is not reasonable for carbide formation.
[0023] Exceeding the silicon content of more than 0.1% negatively affects the processability of the alloy, as well as leads to embrittlement of the alloy due to an increase of silicon silicates content in it.
[0024] Increase of manganese content over 1.0% leads to the appearance of a fusible eutectic, which leads to the destruction of the ingot during pressure processing and reduces the heat resistance of the alloy, as well as leads to a decrease of local corrosion resistance.
[0025] Nickel is stable in HCl even at boiling point. However, in the presence of chlorides, ions of Fe(III) and other oxidizing agents corrosion of nickel and nickelchrome molybdenum alloys is enhanced, the limitation of the iron content of not more than 0.75% is due to this.
[0026] The introduction of titanium in an amount of 0.01-0.06% increases the corrosion resistance in melts of zirconium and hafnium salts, binds residual carbon to carbides and leads to the formation of a sufficient amount of Ni3Ti type intermetallic compound, which, at an operating temperature of 500-700C, positively affects the heat resistance of the alloy. When the titanium content is less than 0.01%, the requirements for corrosion resistance are not met, and the excess of the titanium content above 0.06% leads to a decrease in the processability of the alloy and the formation of undesirable phases due to the reactivity of titanium.
[0027] Aluminium and magnesium in the amount of 0.1-0.2% and 0.005-0.01% are introduced into the alloy to remove residual oxygen, as well as, with regard to aluminium, to form an intermetallic compound of the Ni3Al type, which positively affects the heat resistance of the alloy. When these elements are introduced in amounts less than specified, the necessary removal of residual oxygen is not achieved. If the content of these elements is exceeded, gross non-metallic inclusions are formed.
[0028] When the sulphur content exceeds 0.012% and phosphorus exceeds 0.015%, coarse non-metallic inclusions are formed that adversely affect the ductility of the alloy.
[0029] Under the condition
when the ratio decreases below 46.4, the alloy structure becomes less stable (sigma phase is released), which has a negative effect on plastic characteristics and corrosion resistance.
[0030] In the condition
with a ratio of less than 1.66, a decrease in the corrosion resistance of the alloy occurs.
[0031] The proposed ratio of the elements in the alloy were found experimentally and are optimal, since they allow obtaining the claimed comprehensive technical result. When breaking the ratios of the elements, the properties of the alloy deteriorate, their instability is observed, and the complex effect is not achieved.
[0033] Alloy ingots were smelted in vacuum induction furnaces. The change in the plastic properties of the studied alloys under the influence of temperatures of 550°C and 625°C after long exposure in the furnace for more than 1000 hours was controlled by bending samples to an angle of 90 degrees or more according to GOST 14019-2003. Industrial corrosion cracking resistance tests of alloys were carried out in molten chlorides KCl, AlCl3 + (ZrCl4 HfCl4)
[0034] Table 1 shows the chemical composition of alloy ingots with various compositional options, as well as the prototype alloy. Table 2 shows the results of determining the plastic properties of the alloys indicated in table 1 by bending at an angle of 90 degrees according to GOST 14019-2003. Table 3 presents the results of industrial corrosion cracking resistance tests of the alloys indicated in Table 1 in molten chlorides KCl, AlCl3 + (ZrCl4 HfCl4), 100 hours, at T = 650°C.
[0035] As can be seen from tables 1, 2, the plastic properties of alloy at 550 and 625C with the claimed composition (alloys 1, 2) are higher than the properties of the prototype alloy, alloy 3, not satisfying the claimed composition, has lower plastic characteristics than alloys 1, 2, which leads to the formation of cracks as a result of bending tests according to GOST 14019-2003.
[0036] As it can be seen from table 3, the corrosion rate of alloys (alloys 1, 2) that satisfy the claimed composition is lower than the corrosion rate of the prototype alloy, visual inspection did not reveal the cracks, unlike the prototype alloy. The corrosion rate of alloy 3, which does not satisfy the claimed composition, exceeds the corrosion rate of alloys 1, 2 (however, lower than the corrosion rate of the prototype alloy), visual inspection revealed a crack in the sample.
Table 1 - Chemical composition of the investigated alloys
| Alloy | C | Mn | Si | Mo | Cr | Nb | S | P | Fe | Ti | Al | W | Ni and unavoidable impurities | Ratio (1) | Ratio (2) |
| Alloy 1 | 0.0011 | 0.55 | 00.7 | 13.0 | 23.3 | 0.03 | 0.0028 | 0,01 | 0.54 | ≤0.01 | 0.1 | - | balance | 67.2 | 27.27 |
| Alloy 2 | 0.005 8 | 0.31 | 0,10 | 13.1 | 22.9 | 0.02 | 0.005 | 0.005 | 0.75 | 0.05 | 0.1 | - | balance | 48.0 | 3.45 |
| Alloy 3 | 0.009 | 0.65 | 0.10 | 12.5 | 23.6 | 0.01 | 0.008 | 0.009 1 | 0.84 | 0.04 | 0.1 | - | balance | 42.98 | 1.11 |
| Alloy acc.to prototype | 0.017 | 0.63 | 0.08 | 16.2 | 15.6 | - | 0.006 | 0.009 | 0.45 | - | - | 3.7 | balance | - | - |
Table 2 - Results of determining plastic properties by bending at an angle of 90 degrees
according to GOST 14019-2003
| Alloy | Exposure temperature, °C | |||
| 550°C | 625°C | |||
| Exposure time, h | Samples bending result | Exposure Time, h | Sample bending result | |
| Alloy acc.to prototype | 720 | Sample broken | 720 | Sample broken |
| 1000 | No cracks | 1000 | Crack | |
| 2065 | Crack | 2065 | Crack | |
| Alloy 1 | 720 | No cracks | 720 | No cracks |
| 1000 | No cracks | 1000 | No cracks | |
| 2065 | No cracks | 2065 | No cracks | |
| Alloy 2 | 720 | No cracks | 720 | No cracks |
| 1000 | No cracks | 1000 | No cracks | |
| 2065 | No cracks | 2065 | No cracks | |
| Alloy 3 | 720 | No cracks | 720 | No cracks |
| 1000 | No cracks | 1000 | Crack | |
| 2065 | Crack | 2065 | Crack | |
Table 3 - Results of industrial corrosion cracking resistance tests of alloys in chloride
melts
| KCl, AlCl3 + (ZrCl4 HfCl4), 100 h, at T = 650 C | ||
| Alloy | Visual inspection Cracks after testing | Corrosion rate, mm/year |
| Alloy acc.to prototype | Crack in sample Pit corrosion in a sample up to 0.1-0.2 mm deep | 0.50 |
| Alloy 1 | No cracks Pit corrosion in metal sample up to 0.1-0.2 mm deep | 0.16 |
| Alloy 2 | No cracks Pit corrosion in a sample up to 0.1-0.2 mm deep | 0.21 |
| Alloy 3 | Crack in sample Pit corrosion in a sample up to 0.1-0.2 mm deep | 0.45 |
1. A corrosion-resistant nickel-based alloy containing carbon, silicon, manganese, chromium,
molybdenum, phosphorus, sulphur, iron, nickel and unavoidable impurities, wherein
it additionally contains titanium, aluminium, niobium, magnesium with the following
components ratio, wt.% :
| Carbon | ≤0.006 |
| Silicon | ≤0.1 |
| Manganese | ≤1.0 |
| Chromium | 22.8-24.0 |
| Iron | ≤0.75 |
| Molybdenum | 12.0-14.0 |
| Niobium | 0.01-0.03 |
| Titanium | 0.01-0.06 |
| Aluminium | 0.1-0.2 |
| Magnesium | 0.005-0.01 |
| Phosphorus | ≤0.015 |
| Sulphur | ≤0.012 |
| Nickel and unavoidable impurities | balance. |
2. The alloy according to claim 1, wherein the content of chromium, molybdenum and iron
is related by the ratio:
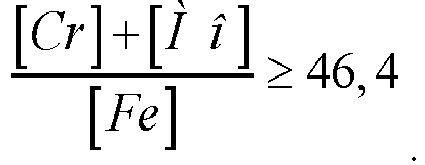
3. The alloy according to claim 1, wherein the content of niobium and carbon is related
by the ratio:
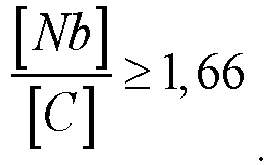
4. The alloy according to claim 1, wherein the content of chromium, molybdenum and iron
is related by the ratio:
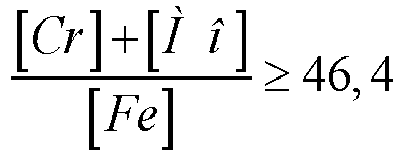
and the content of niobium and carbon is related by the ratio:
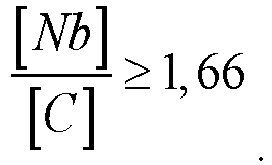
and the content of niobium and carbon is related by the ratio:
REFERENCES CITED IN THE DESCRIPTION
This list of references cited by the applicant is for the reader's convenience only. It does not form part of the European patent document. Even though great care has been taken in compiling the references, errors or omissions cannot be excluded and the EPO disclaims all liability in this regard.
Non-patent literature cited in the description
- M., PROMETEY-SPLAVCorrosion-resistant, heat-resistant and high-strength steels and alloysCatalogue, 2008, 304-306 [0002]
