|
(11) | EP 0 043 680 A1 |
| (12) | EUROPEAN PATENT APPLICATION |
|
|
|
|
|||||||||||||||||||||||||||
| (54) | Process for removing contaminants from a tin surface |
| (57) An aqueous alkaline cleaning solution for removing lubricants or other soiling contaminants
from tin without visibly etching it has a pH of about 11 to 13, imparted by an effective
amount of an alkaline component and also contains, serving as inhibitor, from 0.02
g/i to 0.06 g/l of one or more quinones and/ or polyhydroxybenzenes, these being either
unsubstituted or substituted with halo, alkyl, alkoxy, carboxyl, nitro and/or cyano
substituents. To prevent precipitation of the calcium and/or magnesium ions which
cause water-hardness the solutions desirably contain at least about 0.1 g/l of a polyelectrolyte.
The cleaning process using these solutions is operated at from about 100°F to about
130°F (approx. 38°C to 54°C). A solid cleaning composition or an aqueous concentrate
can be dissolved in water to form the desired solution. |
[0001] This invention relates to an aqueous alkaline cleaning solution and a process employing that solution for cleaning tin surfaces, as well as a solid cleaning composition and a cleaning concentrate for use in the process.
[0002] This invention is particularly concerned to provide a corrosion inhibitor for use in the aqueous alkaline cleaning solution which will deter or inhibit etching of the tin surface. Although the invention is applicable to the removal of contaminants from all kinds of tin surface, it will here be described in connection primarily with the cleaning of tin-plated surfaces, such as tin cans, which have been soiled with organic lubricants of the kind that are applied to the tin surface as drawing aids during cold-forming operations.
[0003] Cleaning is an essential preliminary to many surface-finishing operations. It is for example normally necessary to clean any metal surface prior to corrosion-preventive treatments, and prior to the application of organic finishes to the surface; and cleaning is especially important in the case of metal surfaces to which organic materials have been recently applied as an aid to cold-forming. Such organic lubricating materials must be removed in order to make the metal surface suitably receptive to an organic or inorganic finish.
[0004] A typical and important example of a situation where preliminary cleaning is needed can be found in the manufacture of two-piece, tin-plated, so-called "drawn-and-ironed" cans. Circular bLanks of tin-plated steel (which, due to the high cost of tin, generally have only a thin layer of tin plating) are first cupped and then passed through several drawing dies, so as thus to "iron" the cup - in order thus to form a unitary side-wall and can-bottom structure. Any can produced by this process will normally have a fairly characteristic shape, i.e. it will be a thin-walled, thick- bottomed container having a generally uniform wall thickness; and such cans will be referred to herein as "DI" (drawn-and-ironed) cans.
[0005] The forming operations carried out during the manufacture of DI cans are normally assisted, and indeed the dies and the metallic surface are protected, by the application of lubricants to the tin-plate surface prior to or during the forming operation. The lubricants which are thus applied to the tin surface usually consist of various types of mineral and vegetable oils and heavy metal soaps; and they must be removed if it is desired to have a clean surface in order to assure adhesion of a subsequently applied sanitary lacquer and/or decorative varnish.
[0006] One problem with DI tin-plated containers, which can be serious, is that the drawing operation stretches the tin-plate surface, thereby exposing some of the underlying metal. The underlying metal may very probably be a ferrous metal, such as iron or one of many iron alloys or of a wide variety of steels; and its exposure may easily lead to corrosion. An effective cleaner for tin-plate is one which indeed will provide a water-break-free surface on the tin (a water-break-free surface is one which is sufficiently freed from lubricants, soil, and other contaminants so that it will maintain a continuous film of water) but which will do so without unduly etching the tin and without promoting corrosion of the underlying metal.
[0007] Etching of the tin surface results from chemical attack of the cleaning solution thereon and results in a roughened and dull surface. Inevitably etching also removes some of the corrosion-protective layer of tin from the underlying metal surface, thereby diminishing the anti-corrosion qualities of the tinned surface, and aggravating any corrosion problems arising from exposure of the underlying metal. In the case of say a beverage can, where a smooth, shiny appearance is wanted and maximum safety of the contents is necessary, it is clear that etching and/or corrosion will be highly undesirable.
[0008] Etching and corrosion can be an especially severe problem with conventional cleaners for tin-plate when during the cleaning operation line-stoppage can expose some of the cleaned cans to the cleaner for excessive periods, which can lead to etching and corrosion of the underlying metal surface (no matter whether exposed by stretching or by etching) so severe as to render the cans quite unacceptable for use. Apart from appearance, any corrosion and blemishes on the surface will adversely affect the adhesion of any chemical conversion coating or sanitary lacquer coating that may thereafter be applied to it.
[0009] The cleaners which have been found to be most suitable for producing the desired water-break-free surface on the tin are alkaline cleaners, such as aqueous solutions of alkali metal salts of silicates; phosphates, carbonates and borates- but these all have the undesirable tendency to etch the surface. Efforts have therefore already been made to discover additives which will help to inhibit etching of the tin surface under the alkaline conditions employed.
[0010] Broadly-speaking it is a fair generalization to say that the inhibited alkaline cleaning solutions hitherto suggested have mostly required the use of long periods (for instance up to 15 minutes) and/or relatively high temperatures (at least 140°F [60°C] and often above 150°F [about 65°C]) to achieve satisfactory cleaning. In many instances the suggested inhibitors give rise to waste-disposal and other problems arising from their toxidity or other characteristics.
[0011] United States Patent No. 4,094,701 discloses a process for cleaning a tin surface, without substantially etching it, using an aqueous alkaline solution containing an alkaline component as well as one or more surfactants and, serving as inhibitor, an organic tannin. This solution is sprayed on to the object to be cleaned at elevated temperature for about one to about two minutes. The pH of the cleaner is at least 9, and because higher pH values tend gradually to inactivate the tannin it is preferably between 10 and 13, and most preferably between 10 and 10.5. When in the preferred pH range of say from 9 to 10.5 this cleaner is to be employed at temperatures of 140OF (60°C) and upwards. Thus, while it is true that this United States Patent No. 4,094,701 does disclose a cleaning solution that can be used for cleaning tin surfaces, without etching, at temperatures below boiling in periods of less than two minutes, nevertheless the use of temperatures in excess of 140°F (60oC) is still economically disadvantageous. Moreover the recommended alkaline components include alkali metal borates and phosphates, both of which may give rise to environmental problems and, hence, plant-effluent treatment problems.
[0012] There is thus today still an unfulfilled need for an effective economic and ecologically- acceptable cleaning solution and process which may be used upon tin articles operating at temperatures lower than those previously found to be effective so as to produce a water-break-free surface thereon without etching the tin, no matter whether applied for the necessary minimum periods of about one minute or less or whether by line-stoppage or other accident applied for much longer periods of time than had been intended - without the use of components currently-regarded as environmentally objectionable.
[0013] We have now found that this need can be . largely or even wholly fulfilled by means of the cleaning solutions and processes hereinafter described.
[0014] According to one aspect of this invention there are provided aqueous alkaline cleaning solutions for cleaning lubricants or other soiling contaminants from a tin surface without visibly etching it, these solutions having apH in the range of from about 11 to about 13 and containing effective amounts of an alkaline component and of an inhibitor which inhibitor is or includes one or more substituted or unsubstituted quinones and/or substituted or unsubstituted polyhydroxy-benzenes.
[0015] The term "tin" as used herein refers not merely to pure tin metal but also to alloys wherein tin predominates; and the term "tin surface" refers to the surface of not only articles made of tin but also those plated with tin.
[0016] It will of course be understood that by an "effective amount" of the alkaline component we mean such a concentration as will substantially remove the lubricants or other soiling contaminants, leaving a water-break-free tin surface. Similarly by an "effective amount" of the inhibitor we mean such a concentration as is able substantialy to inhibit etching of the tin surface.
[0017] The term "polyhydroxy-benzenes" is here used to designate otherwise substituted or unsubstituted benzenes which bear at least two hydroxy substituents, in ortho-, meta-or para- positions, and perhaps more than two such hydroxy substituents arranged either symmetrically or asymmetrically.
[0018] According to another aspect of this invention there is also provided a process for cleaning lubricants or other soiling contaminants from a tin surface without visibly etching it, in which the surface is brought into contact for a sufficient time at the chosen temperature with an aqueous alkaline cleaning solution as herein disclosed having a pH in the range of from about 11 to about 13 and containing effective amounts of an alkaline component and of an inhibitor which is or includes one or more substituted or unsubstituted quinones and/or substituted or unsubstituted polyhydroxy-benzenes.
[0019] The inhibitor used in the cleaning solution and process of this invention must be one or more substituted or unsubstituted quinones and/or substituted or unsubstituted polyhydroxy-benzenes. While the nature of the substituents (leaving aside the hydroxy substituents on the polyhydroxy-benzenes) does not seem to be of any fundamental significance, it can be noted for general guidance that such substituents, both upon the polyhydroxy-benzenes and the quinones, can include halo, alkyl, carboxy, nitro, cyano and alkoxy substituents.
[0020] Exemplary quinones and substituted quinones useful as inhibitors in this invention include the substituted and unsubstituted quinones according to general formulae II and III below:
wherein:
R3 and R4, which may be the same or different, each represents a hydrogen atom or an alkyl, alkoxy, hydroxy, halo, nitro, cyano or carboxyl group; and
either R1 and R2, which may be the same or different, each represents an atom or group defined in the same manner as for R3 and R4 or together R1 and R2 constitute an alkyldienyl group which in turn, together with the quinone ring to which they are attached, forms a napthaquinone.
[0021] Exemplary substituted or unsubstituted polyhydroxy-benzene inhibitors, of particular interest for use in this invention, include for instance catechol (ortho-dihydroxy benzene) and/or resorcinol (meta-dihydroxy benzene) and/or hydroquinone (1,4-dihydro--benzoquinone or para-dihydroxy benzene) and/or hydroxy-hydroquinone (1,2,4-trihydroxybenzene and/or 1,3,5-trihydroxybenzene and/or gallic acid (3,4,5-trihydroxybenzoic acid and/or 1,2,4,5-tetrahydroxybenzene.
[0022] Preferred inhibitors for use in this invention include gallic acid (3,4,5-trihydroxybenzoic acid), 1,2,3-benzene-triol and dihydroxybenzenes as well as quinones, such as 1,4--benzenediol and 1,4-benzoquinone.
[0023] The inhibitor must naturally be added in an amount effective to inhibit etching of the tin surface. Generally, the necessary minimum concentration of the inhibitor will increase with the concentration ofthe alkaline component. Moreover, since "soft" water tends to be more corrosive than "hard" water (as will be further discussed below) generally the necessary minimum concentration of inhibitor will tend to be greater when the cleaning solutions are made-up with soft water than when they are made-up with hard water. The concentration of inhibitor in the solution will advantageously be at least about 0.02 g/l; and it will preferably be in the range of from about 0.02 g/l to about 0.06 g/1. There is no objection to the use of concentrations greater than 0.06 g/l, but generally the resultant increase in cost is not repaid by a greater inhibition of etching.
[0024] The addition of a polyelectrolyte to the cleaning solutions of the invention has been found to be beneficial since then the solution may be employed effectively irrespective of the hardness of the water used to make-up the solution.
[0025] Hardness is a characteristic of water, generally accepted to represent the total concentration of calcium and magnesium ions - although other polyvalent cations, seldom present in more than trace amounts, may sometimes also contribute to hardness. The determination of hardness can be carried out by titration, as described in "Standard Methods of Test for Hardness in Water", Designation D 1126-67 (Reapproved 1974) published by the American Society for Testing Materials. Hardness may conveniently be expressed in parts per million (ppm) of calcium carbonate equiva- lent; the higher the value, the greater being the hardness. Usually water having a hardness of less than about 100 ppm will be regarded as "soft", whereas with a hardness about about 100 ppm it will be regarded as "hard". Water-hardness can vary greatly from place to place. In some coastal regions of the United States the hardness may be only about 50 ppm to about 100 ppm, but it may increase to about 300 ppm where the water runs somewhere through limestone deposits, and in some areas of the Midwest of the United States the hardness may be as much as from about 400 ppm to about 500 ppm.
[0026] When the water used to make-up the alkaline aqueous solutions of this invention is rather hard then, unless indeed polyelectrolytes are present, there is a tendency to experience precipitation of the calcium and magnesium ions that cause water hardness. The greater the hardness of the water the greater usually will be the degree of precipitation. Any such precipitation is potentially a problem, since it may result in sediment which is liable, for example, to clog spray nozzles when spraying is the chosen method of application. The use of softer water is moreover not an altogether satisfactory answer to this problem. As hardness decreases, the water may tend to become more corrosive. A polyelectrolyte is therefore a very desirable, though optional, component of the cleaning solutions of this invention.
[0027] Polyelectrolytes are high molecular weight electrolytes; and they are either of natural origin, such as proteins, or they are of a synthetic nature, such as polymerized organic acids. Since the polyelectrolytes in solution do not dissociate to give a uniform distribution of positive and negative ions, as do simple electrolytes, the ions of one sign are bound to the polymer chain. Thus, for instance, the negative charges-may be in the polymer chain, and only positive ions will be free to diffuse through the solvent.
[0028] The polyelectrolytes used in the solutions of this invention can be any of the compounds known to prevent precipitation of the minerals which cause water-hardness, but certain preferences can usefully be borne in mind. Because phosphates may be regarded as environmentally-objectionable, we prefer to use a polyelectrolyte which does not contain phosphates, since then the plant effluent will not contain phosphates. We also prefer to avoid the use of the previously- employed alkali metal tripolyphosphates, because these may have the deleterious effect of promoting etching or de-tinning. Because they do not promote de-tinning and possibly may to some extent inhibit etching the polyelectrolytes which we prefer for use in the cleaning solutions of the present invention are polyelectrolytes such as Gantrez S-95, manufactured by GAF Corporation, and Tamol 960 manufactured by Rohm and Haas Company.
[0029] The polyelectrolytes will preferably be present in a concentration which is effective substantially to prevent precipitation of the calcium and magnesium ions that are the primary cause of water-hardness. Generally, the greater the hardness of the water the greater will be the desired concentration of the polyelectrolyte. We have found that the conentration of polyelectrolyte will preferably be at least about 0.1 g/l, and most advantageously in the range of from about 0.1 g/l to about 1 g/1. There is no objection to the use of concentrations in excess of 1 g/l, but in our experience such higher concentrations do not result in any significant improvement.
[0030] One or more surfactants or wetting agents can usefully be added to the aqueous alkaline cleaning solutions of the present invention, so as to enhance the performance of the cleaning solution in a conventional manner - but the presence or absence of surfactants has no noticeable effect as regards either inhibiting or promoting the etching of the tin surface by the cleaning solution. Supposing a surfactant is added which is not a low-foaming surfactant, then a defoaming agent will also desirably be added - since otherwise foaming can become so serious as to cause delay or even shut-down in a .commercial cleaning line.
[0031] The surfactants and wetting agents employed in the cleaning solutions can for instance be ethoxylated straight-chain alcohols and octyl or nonyl phenoxy polyethoxyethanol. Nonionic surfactants are usually preferred since they are low-foaming.
[0032] The cleaning solutions will preferably contain at least about 0.2 g/l of the surfactant(s); particularly preferred concentrations are in the range of from about 0.2 g/l to about 0.5 g/l.
[0033] As already indicated, the cleaning solution must have a pH value (determined at a solution temperature of about 27°C) within the range of from about 11 to about 13; and will preferably have a pH value within the range of from about 12 to about 12.8.
[0034] The alkaline component used in the cleaning solution and process of this invention may be of any kind known to produce an alkaline solution when dissolved in water; but it will preferably not be an environmentally-objectionable one. The alkaline component therefore can advantageously be one or a mixture of more than one alkali metal hydroxides and/or carbonates and/or silicates and/or ammonium hydroxides and/or carbonates. A particularly preferred alkaline component is a mixture of sodium carbonate, sodium metasilicate and either sodium hydroxide or potassium hydroxide or both.
[0035] The alkaline component must naturally be present in a concentration which will be effective substantially to remove lubricants or other such soiling contaminants. The minimum effective amount may be readily determined in the conventional manner. The concentration of alkaline component in the solution will preferably be at least about 2 g/l, and more advantageously will be in the range of from about 3g/l to about 5 g/l. Greater concentrations may be employed, but the benefit to be gained thereby is generally not significant.
[0036] Tin surfaces may be cleaned by contacting them with the aqueous alkaline cleaning solutions of the present invention for a sufficient time at the chosen temperature to remove lubricants or other such soiling contaminants therefrom, without visibly etching the tin surface. Usually the solution temperature should be at least about 100°F (380C); and it will preferably be in the range of from about 100°F to about 130°F (38° to 54°C) and above all from about 115°F to about 1250F (460 to 520C). The optimum temperature may tend to be somewhat higher for solutions with low concentrations of alkaline components than for solutions with higher concentrations thereof - or, put another way, the optimum treatment temperature tends somewhat to vary inversely with the pH of the cleaning solution.
[0037] The necessary period of treatment will generally depend on the method of application. Spraying is the preferred method, and when used the treatment time will tend to decrease as the spraying pressure increases. Operating at currently conventional spraying pressures of about 25 psig (pounds per square inch, guage pressure) to about 35 psig (1.76 to 2.46 kg/cm2) it should be found that treatment times of from about 40 seconds up to about one minute may be sufficient to produce a water-break-free surface. If one employs the already- known but little used spraying pressures of 60 psig to 100 psig (4.22 to 7.03 kg/cm2) then the treatment times may be reduced, perhaps to as little as from about 1 to 5 seconds.
[0038] The treatment time may need to be increased the longer the interval between manufacture of the tin-plated metal article and the cleaning thereof. With drawn-and-ironed tin-plated steel cans, an interval between manufacture and cleaning of as little as fifteen minutes may necessitate a noticeably increased cleaning time. The necessary treatment time may also vary depending on the type of lubricant used in the manufacturing process; some being more readily removed than others.
[0039] In order to discover the extent to which the cleaning solutions of this invention will inhibit etching we have used treatment times of up to about 30 minutes without visibly etching the tin; but in actual practice it is of course recommended to use the shortest treatment time which is effective to produce a water-break-free surface without etching the tin.
[0040] After the tin surface has been cleaned with the cleaning solution, the clean surface will generally be rinsed at least once with tap water, and then may advantageously be rinsed with deionized water and dried at an elevated temperature, preferably one in the range of from about 350° to about 400°F (1770 to 205°C).
[0041] It is convenient to make up the cleaning solutions of the present invention from either an aqueous concentrate or a solid cleaning composition, which can simply be added to water to produce the desired aqueous alkaline cleaning solutions.
[0042] Such an aqueous concentrate may advantageously be formulated so that when added to water at a concentration in the range of from about 0.5% to about 2% by volume, and preferably in the range of from about 1% to about 2% by volume, it produces an aqueous alkaline cleaning solution having a pH of about 11 to about 13, and comprises an alkaline component in an amount effective substantially to remove contaminants from the tin surface, as well as one of the herein-defined inhibitors in an amount effective to inhibit etching of the surface. The alkaline component will preferably constitute up to about 770 grams per litre, and advantageously from about 250 to about 500 grams per litre of the concentrate.
[0043] The inhibitor will preferably constitute at least about 2 grams per litre, and advantageously from about 2 to about 6 grams per litre of the concentrate. The concentrate optionally but desirably will also contain polyelectrolyte(s) and/ or surfactant(s). The polyelectrolyte will desirably be present in a concentration within the range of from about 10 to about 20 grams per litre.
[0044] Alternatively a solid cleaning composition may advantageously be formulated so that when added to water at a concentration of from about 3 g/l to about 7 g/l, and preferably from about 4.6 g/l to 6.7 g/1, it will produce an aqueous alkaline cleaning solution having a pH of about 11 to about 13 and contains an effective amount of an alkaline component and an effective amount of an inhibitor as defined above. The alkaline component will preferably constitute at least about 15% by weight of the composition. The inhibitor will preferably constitute at least about 0.1% by weight, and most advantageously from about 0.5% to about 2% by weight of the composition. Moreover the composition optionally but desirably will also contain a polyelectrolyte, present preferably in a concentration of up to about 10% by weight, and most advantageously in a concentration of from about 3% to about 10% by weight of the composition.
[0045] The balance (if any)of the cleaning composition may consist of diluents and the like, that is to say compounds which can be regarded as inert with respect to any possible influence, either positive or negative, upon the cleaning and non-etching properties of the cleaning solutions of the invention. A typical but merely illustrative example of such compounds is for instance sodium sulphate.
[0046] In order that the invention may be well understood, it will now be described in more detail, but only by way of illustration, in the following examples:
EXAMPLES
[0047] It should first be noted that, in each of the following Examples, sets of "drawn-and-ironed" tin-plated steel cans (previously defined and hereinafter called DI cans) were contacted with the specified cleaning solution within 24 hours of their manufacture, and without any other treatment prior to contact with the cleaning solutions of the invention.
[0048] The cleaning solutions were made up from solid cleaning compositions having the components indicated in Table I below present in the percentages there shown. Each such composition is identified in Table I and elsewhere by a number and the subscript "c". The solutions were made up by dissolving a specific amount,. in grams, of each composition as indicated in Table II below to 6 litres of water, to form aqueous alkaline cleaning solutions having the concentrations of components (including surfactants) indicated in Table III below. Each such cleaning solution is identified in Table III and elsewhere by the number of the composition from which it has been made, coupled with the subscript "s".
[0049] In each of the Examples the sets of cans were contacted with the cleaning solution by spraying them with the solutions at about 25psi (1.76 kg/cm2) for the periods and at the temperatures there stated; and afterwards the cans were then rinsed with water, and visually examined for etching and appearance.
Example 1
[0050] Sets of DI cans were sprayed, one set per solution, with cleaning solutions 1s, 2s, 3s and 4s, for 10 minutes at 123°F (about 51°C). The results obtained are summarized in Table IV as follows :
Example 2
[0051] Sets of DI cans were sprayed, one set per test, with cleaning solution 5s for the times and at the temperatures indicated; and the results obtained are summarized in Table V as follows :
Example 3
[0052] Sets of DI cans were sprayed with cleaning solutions 6s, 7s, 8s and 9s, one set per test, for the times and at the temperatures indicated. The results are summarized in Table VI.
Example 4
[0053] Sets of DI cans were sprayed with cleaning solutions 10s, 11s, and 12s, one set per test, for the times and at the temperatures indicated; and the results observed are summarized in Table VII as follows :
Example 5
[0054] Aqueous concentrates were prepared having the concentrations of components indicated in Table VIII. These concentrates are designated 13 aq and 14 aq.
[0055] Aqueous alkaline cleaning solutions 13s and 14s were formulated, respectively, by diluting 11.4 millilitres of aqueous concentrate 13 aq to 1 litre with tap water, and by diluting 10 millilitres of aqueous concentrate 14 aq to 1 litre with deionized water. Surfactants were added in the concentrations indicated. The cleaning solutions had the concentrations of components indicated in Table VIII, as follows :
[0056] Sets of DI cans were sprayed with cleaning solutions 13s and 14s, one set per test, for the times and at the temperatures indicated; and the results obtained are summarized in Table IX as follows :
Example 6
1. An aqueous alkaline cleaning solution for removing lubricants or other soiling
contaminants from a tin surface without visibly etching it, said solution having a
pH in the range of from about 11 to about 13 and containing effective amounts of an
alkaline component and of an inhibitor, characterized in that the inhibitor is or
includes one or more substituted or unsubstituted quinones and/or substituted or unsubstituted
polyhydroxy- benzenes.
2. A cleaning solution as claimed in claim 1, characterized in that the inhibitor
is or includes a substituted quinone and/or a substituted polyhydroxy-benzene wherein
the substituent(s), besides the hydroxy substituent on the benzene ring, include a
halo, alkyl, alkoxy, carboxyl, nitro and/or cyano substituent.
3. A cleaning solution as claimed in claim 1 or claim 2, characterized in that the
inhibitor is or includes a substituted or unsubstituted quinone conforming to one
of the general formulae
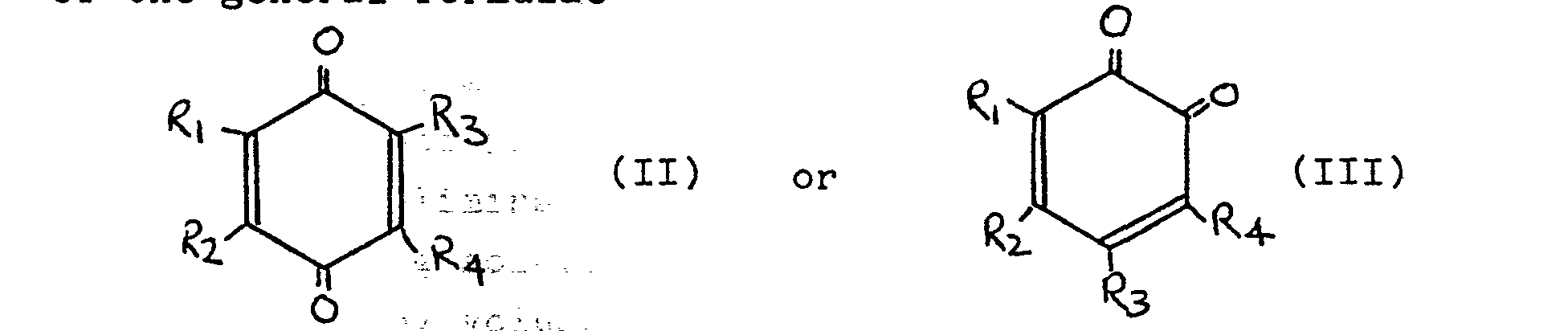
in which R3 and R4, which may be the same or different, each represents a hydrogen atom or a hydroxy, halo, alkoxy, alkyl, carboxyl, nitro or cyano group; and either R1 and R2 which may be the same or different each represents at atom or group as defined for R3 and R4 or together R1 and R2 constitute an alkyldienyl group which in turn, together with the quinone ring to which they are attached, forms a naphthaquinone.
in which R3 and R4, which may be the same or different, each represents a hydrogen atom or a hydroxy, halo, alkoxy, alkyl, carboxyl, nitro or cyano group; and either R1 and R2 which may be the same or different each represents at atom or group as defined for R3 and R4 or together R1 and R2 constitute an alkyldienyl group which in turn, together with the quinone ring to which they are attached, forms a naphthaquinone.
4. A cleaning solution as claimed in claim 3, characterized in that the inhibitor
is or includes 1,4-benzoquinone and/or 1,2-benzoquinone and/or α-naphthaquinone and/or
β-naphthaquinone.
5. A cleaning solution as claimed in claim 1 or claim 2, characterized in that the
inhibitor is or includes one of the following substituted or unsubstituted polyhydroxy-
benzenes, namely catechol (ortho-dihydroxy benzene) and/or resorcinol (meta-dihydroxy
benzene) and/or hydroquinone (1,4-dihydrobenzoquinone or para-dihydroxy. benzene)
and/or hydroxy-hydroquinone (1,2,4-trihydroxy- benzene) and/or 1,3,5-trihydroxy-benzene
and/or gallic acid (3,4,5-trihydroxy benzoic acid) and/or 1,2,4,5-tetrahydroxy-benzene.
6. A cleaning solution as claimed in any of the preceding claims, characterized in
that the inhibitor is present in a concentration in the range of from about 0.02 g/1
to about 0.06 g/1.
7. A cleaning solution as claimed in any of the preceding claims, characterized in
that it also includes a polyelectrolyte in a concentration effective substantially
to prevent precipitation of calcium and/or magnesium ions causing water-hardness.
8. A cleaning solution as claimed in claim 7, characterized in that the polyelectrolyte
is present in a concentration of at least about 0.1 g/l.
9. A cleaning solution as claimed in any of the preceding claims, characterized in
that the alkaline component therein consists of one or a mixture of more than one
alkali metal hydroxides and/or alkali metal carbonates and/or alkali metal silicates
and/or ammonium hydroxide and/or ammonium carbonate.
10. A process for removing lubricants or other soiling contaminants from a tin surface
without visibly etching it, in which the surface is brought into contact for a sufficient
time at the chosen temperature with an aqueous alkaline cleaning solution, as claimed
in any of the preceding claims, characterized in that the temperature employed is
in the range of from about 100° to about 1300F (approx. 38°C to 54°C).
11. A process as claimed in claim 10, characterized in that it includes the preliminary
step of making-up the aqueous alkaline cleaning solution by dissolving from about
3 g/1 to about 7 g/1 of a solid cleaning composition in water, at least 15% by weight
of the composition being formed by the alkaline component and at least about 0.1%
by weight of the composition being formed by the inhibitor, said composition also
optionally including a plyelectrolyte in a proportion effective substantially to prevent
precipitation of calcium and magnesium ions in hard water.
12. A process as claimed in claim 10, characterized in that it includes the preliminary
step of making-up the aqueous alkaline cleaning solution by dissolving from about
0.5% to about 2% by volume of an aqueous concentrate in water, said concentrate containing
up to 770 g/1 of the alkaline component and at least about 2 g/1 of the inhibitor,
as well as optionally a polyelectrolyte in a proportion effective substantially to
prevent precipitation of calcium and magnesium ions in hard water.
13. A solid cleaning composition, for use in the process of claim 11, characterized
in that it comprises at least 15% by weight of an alkaline component and at least
about 0.1% by weight of an inhibitor which is one or more substituted or unsubstituted
quinones and/or substituted or unsubstituted polyhydroxybenzenes, as well as optionally
an effective amount of a polyelectrolyte.
14. An aqueous concentrate, for use in the process of claim 12, characterized in that
it comprises up to 770 g/1 of an alkaline component and at least about 2 g/1 of an
inhibitor which is one or more substituted or unsubstituted quinones and/or substituted
or unsubstituted polyhydroxybenzenes, as well as optionally an effective amount of
a polyelectrolyte.
