|
(11) | EP 0 529 811 A1 |
| (12) | EUROPEAN PATENT APPLICATION |
|
|
|
|
|||||||||||||||||||||||||||
| (54) | Light-sensitive silver halide color photographic material |
| (57) A light-sensitive silver halide color photographic material is disclosed. The material
comprises a support and provided thereon photographic component layers comprising
a blue-sensitive silver halide emulsion layer, a green-sensitive silver halide emulsion
layer and a red-sensitive silver halide emulsion layer, wherein at least one green-sensitive
silver halide emulsion layer contains a colored magenta coupler represented by the
following Formula CM-1 and at least one of said photographic component layers contains
a heterocyclic ring containing fog restrainer:
|
FIELD OF THE INVENTION
[0001] The present invention relates to a light-sensitive silver halide color photographic material. More particularly it relates to a light-sensitive color photographic material that may cause less increase in fog after storage when light-sensitive materials are stored in a high-temperature high-humidity environment, and may cause less variation between different types of printers used.
BACKGROUND OF THE INVENTION
[0002] In light-sensitive color photographic materials, yellow, magenta and cyan dyes formed from couplers do not necessarily have ideal absorption characteristics. For example, it is common for magenta images not only to absorb the inherent green light but also to absorb slightly blue light. This causes a distortion in color reproduction. In order to remove such a distortion in color reproduction, a coupler having been colored in yellow or magenta is used before the coupling reaction with an oxidized product of an aromatic primary amine. The former is what is called a colored magenta coupler, and the latter, a colored cyan coupler.
[0003] An auto-masking method making use of such colored couplers is described in detail in, for example, J. Phot. Soc. Am., 13, 94 (1947), J. Opt. Soc. Am., 40, 166 (1950) or J. Am. Chem. Soc., 72, 1533 (1950).
[0004] As colored magenta couplers having a main absorption in the blue light region, U.S. Patents No. 2,428,054 and 2,449,966 disclose 1-phenyl-3-acylamino-4-phenylazo-5-pyrazolone; U.S. Patent No. 2,763,552, those having a 4-methoxyallylazo group; U.S. Patent No. 2,983,608, 1-phenyl-3-anilino-4-phenylazo-5-pyrazolone; U.S. Patents No. 3,519,429 and No. 3,615,506, those having a naphthylazo group; U.S. Patent No. 1,044,778, those having a water-soluble group; U.S. Patent No. 3,476,564 and Japanese Patent Publications Open to Public Inspection [hereinafter referred to as Japanese Patent O.P.I. Publication(s)] No. 123625/1974, No. 131448/1974 and No. 52532/1979, those having a hydroxyphenylazo group; Japanese Patent O.P.I. Publication No. 42121/1977, those having an acylaminophenylazo group; Japanese Patent O.P.I. Publication No. 102723/1977, those having a substituted alkoxyphenylazo group; and Japanese Patent O.P.I. Publication No. 63016/1978, those having a thiophenylazo group.
[0005] These colored magenta couplers, however, have, for example, so small molar absorption coefficient that they must be added in a large quantity and it is difficult to adjust the main absorption to the desired region. For another example, they have so low a development activity that they have a small masking effect, or, even though they have a high development activity, they tend to cause fogging. For still another example, they have a low stability to light, heat and humidity, and magenta dyes formed upon reaction with a color developing agent have an absorption wavelength region shorter than the desired region. Thus, none of them are preferable with much satisfaction. Under existing circumstances, their use in combination of plural kinds barely maintains their performance. Particularly in recent years, colored magenta couplers are required to have much higher performance since silver halide emulsions comprised of high-sensitivity fine grains or magenta couplers having high color forming properties have become prevalent.
[0006] In particular, in recent years, it has become known that a mismatch of hues of finished color prints may occur because of difference in type of the machinery for printing (hereinafter "printer(s)") to be used (hereinafter "variation between different types of printers"). As a cause thereof, it has become clear that this is caused by the color tones of dyes obtained from colored magenta couplers used in color negative films.
[0007] The variation between different types of printers can be much better prevented when colored magenta couplers as disclosed in Japanese Patent O.P.I. Publication No. 16939/1992 are used. However, studies made by the present inventors have revealed that use of the colored magenta couplers may cause an increase in fog or a decrease in sensitivity when light-sensitive materials are stored in a high-temperature high-humidity environment before exposure (hereinafter "deterioration of raw storage stability"), which is a problem to be solved.
SUMMARY OF THE INVENTION
[0008] An object of the present invention is to provide a light-sensitive silver halide color photographic material that may cause less increase in fog after storage when light-sensitive materials are stored in a high-temperature high-humidity environment, and may cause less variation between different types of printers used.
[0009] The above object of the present invention can be achieved by the following light-sensitive silver halide color photographic material.
[0010] A light-sensitive silver halide color photographic material comprising a support and provided thereon photographic component layers comprising a blue-sensitive silver halide emulsion layer, a green-sensitive silver halide emulsion layer and a red-sensitive silver halide emulsion layer, wherein at least one green-sensitive silver halide emulsion layer contains a colored magenta coupler represented by the following Formula CM-I and at least one of said photographic component layers contains a heterocyclic ring containing fog restrainer.
wherein R₁ represents a substituent; R₂ represents an acylamino group, a sulfonamido group, an imido group, a carbamoyl group, a sulfamoyl group, an alkoxy group, an alkoxycarbonyl group or an alkoxycarbonylamino group; R₃ represents a halogen atom or an alkoxyl group; and m represents an integer of 0 to 5, and n, 0 to 4.
[0012] In Formula CM-I, the substituent represented by R₁ may include, for example, an alkyl group, an alkoxy group an aryl group, an acylamino group, a sulfonamido group, a hydroxyl group, a halogen atom, an alkoxycarbonyl group, an acyl group, a carbamoyl group, a sulfamoyl group and a carboxyl group. These groups may further have a substituent. R₁ may preferably be an alkyl group, an alkoxy group, a hydroxyl group or an acylamino group, and most preferably an alkoxy group.
[0013] The acylamino group represented by R₂ may include, for example, 2,4-di-t-pentylphenoxyacetamido group and 4-(2,4-di-t-pentylphenoxy)butanamido group. The sulfonamido group may include, for example, a 4-dodecyloxyphenylsulfonamido group. The imido group may include, for example, an octadecenylsuccinimido group. The carbamoyl group may include, for example, a 4-(2,4-di-t-pentylphenoxy)butylaminocarbonyl group. The sulfamoyl group may include, for example, a tetradecanesulfamoyl group. The alkoxy group may include, for example, a methoxy group, an ethoxy group and an octyloxy group. The alkoxycarbonyl group may include, for example, a tetradecaneoxycarbonyl group. The alkoxycarbonylamino group may include, for example, a dodecyloxycarbonylamino group. R₂ may preferably be an acylamino group substituted on the para position relative to R₃.
[0014] The halogen atom represented by R₃ may include, for example, a chlorine atom, a bromine atom and a fluorine atom. The alkoxyl group may include, for example, a methoxy group and a dodecyloxy group. R₃ may preferably be a chlorine atom. Letter symbol m may preferably be 1 or 2, and n may preferably be 1.
[0015] Examples of the compound represented by Formula CM-I according to the present invention are shown below. The present invention is by no means limited by these.
[0016] The colored magenta coupler of the present invention, represented by Formula CM-I, can be synthesized by what is called the diazocoupling reaction conventionally carried out, and can be synthesized by, for example, the method disclosed in Japanese Patent Examined Publication No. 6540/1981. More specifically, an aniline derivative is formed into a diazo compound in water, water-containing alcohol or water-containing acetone at 0 to -10°C using 1- to 5-fold mole of concentrated hydrochloric acid and 1- to 1.2-fold mole of sodium nitrite, and the resulting solution is added at -5 to -10°C to a pyridine solution having been separately prepared, containing a magenta coupler in an amount equimolar to the above aniline derivative. Thus the desired colored magenta coupler can be obtained.
[0017] Specific synthesis examples of of the colored magenta coupler represented by Formula CM-I are shown below.
Synthesis Example 1 (Synthesis of CM-7)
[0018] In 3 ml of concentrated hydrochloric acid and 18 ml of water, 1.4 g of 3,4-diethoxyaniline was dissolved after heating once, followed by cooling to -3°C. To the resulting solution, 5.3 ml of aqueous 10% sodium nitrite solution was added to form the 3,4-diethoxyaniline into a diazo compound, which was stirred for 20 minutes at -3°C, followed by addition of 0.1 g of urea to decompose excess nitrite. Separately, 5.2 g of 1-(2,3,4,5,6-pentachlorophenyl)-3-(2-chloro-5-tetradecaneamidoanilino)-5-pyrazolone was dissolved in 100 ml of pyridine, followed by cooling to -5 to -10°C. To the resulting solution the diazonium salt solution previously prepared was slowly added with stirring.
[0019] After 3 hours, the reaction mixture was poured into 400 ml of ice water containing 100 ml of concentrated hydrochloric acid. Crystals formed were filtered, washed with water and then dried, which were thereafter recrystallized from a solution formed of a mixture of ethyl acetate and acetonitrile, to give 5.5 g of CM-7.
Synthesis Example 2 (Synthesis of CM-13)
[0020] In 3 ml of concentrated hydrochloric acid and 20 ml of water, 1.0 g of 4-methoxyaniline was dissolved after heating once, followed by cooling to -3°C. To the resulting solution, 5.3 ml of aqueous 10% sodium nitrite solution was added to form the 4-methoxyaniline into a diazo compound, which was stirred for 20 minutes at -3°C, followed by addition of 0.1 g of urea to decompose excess nitrite.
[0021] Separately, 5.6 g of 1-(2,3,4,5,6-pentachlorophenyl)-3-{2-chloro-5-[α-(2,4-di-t-amylphenoxy)butaneamido] anilino}-5-pyrazolone was dissolved in 100 ml of pyridine, followed by cooling to -5 to -10°C. To the resulting solution the diazonium salt solution previously prepared was slowly added with stirring. After 3 hours, the reaction mixture was poured into 400 ml of ice water containing 100 ml of concentrated hydrochloric acid. Crystals formed were filtered, washed with water and then dried, which were thereafter recrystallized from a solution formed of a mixture of ethyl acetate and acetonitrile, to give 5.1 g of CM-13.
[0023] The colored magenta coupler of the present invention, represented by Formula CM-I, may be used alone or in combination of two or more kinds. In usual instances, the compound is used in combination with one or more kinds of substantially non-dye forming magenta couplers in view of the principle of the automasking.
[0024] The heterocyclic ring containing fog restrainer used in the present invention will be described below. The heterocyclic ring containing fog restrainer refers to a compound having a heterocyclic ring among compounds used for the purposes of preventing fog from occurring during the manufacture, storage or photographic processing of light-sensitive materials or stabilizing photographic performances.
[0025] The heterocyclic ring includes, for example, imidazoles, triazoles, tetrazoles, thiadiazoles, oxadiazoles, pyridines, pyrimidines, benzoimidazoles, benzotriazoles, indazoles, benzothiazoles, benzoxazoles and azaindenes.
[0026] These heterocyclic rings may be substituted with a usual organic group. Such an organic group may include, for example, an alkyl group, a heterocyclic group, an acyl group, an alkoxyl group, a carboxyl group, alkoxycarbonyl group, an amino group, an amido group, a carbamoyl group, a ureido group, a sulfo group, a sulfonamido group, a sulfamoyl group, an alkylthio group, a mercapto group, a hydroxyl group, a nitro group and a halogen atom.
[0027] Examples of the heterocyclic ring containing fog restrainer preferably used in the present invention (hereinafter "restrainer of the present invention" are shown below. The present invention is by no means limited by these.
[0028] Some of these compounds are commercially available. These compounds can also be synthesized according to the methods disclosed, for example, in U.S. Patent No. 3,259,976 and Japanese Patent O.P.I. Publications No. 14836/1982, No. 167023/1982, No. 95728/1983 and No. 68732/1984.
[0029] To incorporate the restrainer of the present invention in the silver halide emulsion layer according to the present invention, it may be dissolved in water or an organic solvent freely miscible in water as exemplified by methanol and ethanol and then added to the intended layer. The restrainer of the present invention may be used alone, or may be used in combination with other type of restrainer of the present invention or a fog restrainer other than the restrainer of the present invention.
[0030] As to the time at which the restrainer of the present invention is added, the restrainer may be added at any time, i.e., before the formation of silver halide grains, during the formation of silver halide grains, in the course from completion of the formation of silver halide grains till initiation of chemical ripening, during chemical ripening, at the time of completion of chemical ripening, or in the course from completion of chemical ripening until coating. It may preferably be added during chemical ripening, at the time of completion of chemical ripening, or in the course from completion of chemical ripening until coating. It may be added at one time in its entirety, or added dividedly at several times.
[0031] As to the location to which the restrainer is added, it may be directly added to a silver halide emulsion when prepared or to a silver halide emulsion coating solution. Alternatively, it may be added to a coating solution for an adjacent non-sensitive hydrophilic colloid layer so that it is diffused during multi-layer coating, into the silver halide emulsion layer according to the present invention.
[0032] On its amount, there are no particular limitations. In usual instances, it may be added in an amount ranging from 1 × 10⁻⁶ mol to 1 × 10⁻¹ mol, and preferably from 1 × 10⁻⁵ mol to 1 × 10⁻² mol, per mol of silver halide.
[0033] As silver halides for the silver halide emulsions used in the light-sensitive silver halide photographic material of the present invention, any of those used in conventional silver halide emulsions can be used, including silver bromide, silver iodobromide, silver iodochloride, silver chlorobromide and silver chloride.
[0034] Silver halide grains used in the silver halide emulsions may be those having uniform distribution of silver halide in each grain, or may be those comprised of a grain with a layer structure having a difference in silver halide composition between its inside and its surface layer.
[0035] The silver halide grains may be comprised of a grain on the surface of which a latent image is mainly formed, or a grain in the inside of which a latent image is mainly formed.
[0036] The silver halide emulsions used may have any grain size distribution. Emulsions having a broad grain size distribution (called polydisperse emulsions) may be used, or emulsions having a narrow grain size distribution (called monodisperse emulsions) may be used alone or in the form of a mixture of two or more kinds. A mixture of the polydisperse emulsion and the monodisperse emulsion may also be used.
[0037] The silver halide emulsions may be used in the form of a mixture of two or more kinds of silver halide emulsions separately formed.
[0038] The silver halide grains used in the present invention may be chemically sensitized by, e.g., sulfur sensitization, selenium sensitization, reduction sensitization or noble metal sensitization.
[0039] The silver halide grains used in the present invention may be spectrally sensitized using dyes known as spectral sensitizers in the photographic industrial field.
[0041] As binders or protective colloids used in the emulsions and others of the light-sensitive silver halide photographic material of the present invention, it is advantageous to use gelatin. Besides, it is possible to use hydrophilic colloids such as gelatin derivatives, graft polymers of gelatin with other macromolecules, proteins, sugar derivatives, cellulose derivatives and homopolymer or copolymer synthetic hydrophilic polymeric substances.
[0042] The emulsion layers and other hydrophilic colloid layers of the light-sensitive silver halide photographic material of the present invention are hardened using alone or in combination hardening agents capable of cross-linking binder or protective colloid molecules to increase film strength.
[0043] The silver halide emulsions may also contain plasticizers, dispersions of water-soluble or sparingly water-soluble synthetic polymers (latexes), and so forth.
[0044] Other couplers may be used in the light-sensitive photographic material of the present invention. It is possible to use competing couplers having the effect of color correction, and compounds capable of releasing photographically useful fragments such as a development accelerator, a bleaching accelerator, a developer, a silver halide solvent, a toning agent, a hardening agent, a fogging agent, an antifoggant, a chemical sensitizer, a spectral sensitizer and a desensitizer upon coupling with an oxidized product of a developing agent.
[0045] As yellow dye forming couplers, known acylacetoanilide type couplers may preferably be used. Of these, it is advantageous to use benzoylacetoanilide compounds and pivaloylacetanilide compounds.
[0046] As magenta dye forming couplers, it is possible to use 5-pyrazolone type couplers, pyrazoloazole type couplers, pyrazolobenzimidazole type couplers, open-chain acylacetonitrile type couplers and indazole type couplers.
[0048] In order to incorporate the coupler in the light-sensitive material, known techniques used in conventional couplers can be applied. It is preferred to dissolve the coupler in a high-boiling solvent optionally using a low-boiling solvent in combination, disperse it in the form of fine particles, and add them to the silver halide emulsion according to the present invention. Here, a hydroquinone derivative, an ultraviolet absorbent and an anti-fading additive may also be optionally used in combination.
[0049] The light-sensitive silver halide photographic material of the present invention may be provided with auxiliary layers such as a filter layer, an anti-halation layer and an anti-irradiation layer. These layers and/or emulsion layers may be incorporated with dyes capable of flowing out of the light-sensitive material or being bleached during photographic processing.
[0050] Matting agents, lubricants, image stabilizers, ultraviolet absorbents, optical brightening agents, surface active agents, development accelerators, development restrainers and bleaching accelerators may be added to the light-sensitive silver halide photographic material of the present invention.
[0051] The photographic emulsion layers and other layers of the light-sensitive silver halide photographic material of the present invention may be provided on a baryta paper, a paper support laminated with an α-olefin polymer or the like, a paper support with an α-olefin layer from which the α-olefin layer is readily separable, a flexible reflective support made of synthetic paper, a film comprised of a semi-synthetic or synthetic polymer such as cellulose acetate, cellulose nitrate, polystyrene, polyvinyl chloride, polyethylene terephthalate, polycarbonate or polyamide, a reflective support coated with a white pigment, or a rigid body such as glass, metal or ceramics. They may also be provided on a single weight type reflective support of 120 to 160 µm thick.
[0052] In order to obtain dye images in the case where the light-sensitive silver halide photographic material of the present invention contains couplers, the light-sensitive material may be exposed to light followed by conventionally known color photographic processing.
[0053] In the present invention, after color developing, the light-sensitive material may be immediately processed using a processing solution having a bleaching ability and a processing solution having a fixing ability. Alternatively, it may be processed using a processing solution having both a bleaching ability and a fixing ability, i.e., what is called a bleach-fixing solution. As a bleaching agent used for bleaching, a metal complex salt of an organic acid is used.
[0054] After fixing, washing is usually carried out. In place of the washing, stabilizing may be carried out, or both the steps may be used in combination.
EXAMPLES
[0055] The present invention will be specifically described below by giving examples. Embodiments of the present invention are by no means limited to these.
Example 1
[0056] On a triacetyl cellulose film support, layers each having the composition as shown below were formed in order of the stated layers to prepare a multi-layer light-sensitive color photographic material, sample 1.
[0057] In the following, the amount of each compound added in the light-sensitive silver halide color photographic material is indicated as gram number per 1 m² unless particularly noted. The amounts of silver halide and colloidal silver are in terms of silver weight. Those of spectral sensitizers are each indicated as molar number per mol of silver.
First layer: Anti-halation layer (HC)
[0058]
- Black colloidal silver
- 0.15
- Ultraviolet absorbent (UV-1)
- 0.20
- Colored cyan coupler (CC-1)
- 0.02
- High-boiling solvent (Oil-1)
- 0.20
- High-boiling solvent (Oil-2)
- 0.20
- Gelatin
- 1.6
Second layer: Intermediate layer (IL-1)
Fifth layer: Intermediate layer (IL-2)
Eighth layer: Yellow filter layer (YC)
[0061]
- Yellow colloidal silver
- 0.1
- Additive (SC-1)
- 0.12
- High-boiling solvent (Oil-2)
- 0.15
- Gelatin
- 1.0
[0062] Coating aid Su-2, dispersion aid Su-1, hardening agent H-1, dyes AI-1 and AI-2 were appropriately added to each layer in addition to the above composition.
[0063] Emulsions used in the above sample are as follows, all of which are internally iodide-rich monodisperse emulsions.
Em-1:
Em-2:
Em-3:
Em-4:
Em-5:
[0069] The procedure for the sample 1 was repeated to produce samples 2 to 18, except that the colored magenta couplers used in the sixth layer and the seventh layer of Sample 1 were respectively replaced with colored magenta couplers as shown in Table 1, and a fog restrainer was added to each silver halide emulsion layer in an amount of 0.1 mmol per mol of silver as shown in Table 1,.
[0070] Using the samples 1 to 18 thus prepared and a camera (KONICA FT-1 MOTOR; manufactured by Konica Corporation), a color checker (manufactured by Macbeth Corporation) was photographed. Thereafter the following photographic processing was carried out.
- Color developing solution -
- Bleaching solution -
- Fixing solution -
- Stabilizing solution -
[0075]
Made up to 1 liter by adding water, and thereafter adjusted to pH8.5 using ammonia water or 50% sulfuric acid.
[0076] The samples obtained were printed using printer-A so as for the gray areas in the color checker to be in a gray color with a reflectance of 18%. Print samples 1A to 18A were thus prepared.
[0077] Next, using printer-B having a green region detector different from that of the printer-A, samples were printed under the same conditions as the printing using the printer-A. Print samples 1B to 18B were thus prepared. The variation between different types of printers were visually judged.
[0078] The samples 1 to 18 each were also left to stand for 7 days in an environment of a temperature of 55°C and a relative humidity of 80% to carry out a raw storage stability test under accelerated aging. Thereafter, the samples thus aged and samples having been put to refrigerated storage were exposed to white light through a step wedge for sensitometry and then processed according to the processing steps described above. Thereafter, sensitometry was made using green light to determine fog and sensitivity of the refrigeratedly stored samples (the fresh performance) and the acceleratedly aged samples. The sensitivity is determined as a reciprocal of the amount of exposure that is necessary for giving a density of fog + 0.3 and is indicated as a relative value assuming the sensitivity of refrigeratedly stored sample 1 as 100. Results obtained are shown together in Table 1.
[0079] As is clear from Table 1, sample 1 outside the scope of the present invention has good raw storage stability but shows a very large variation between different types of printers. The sample 2, in which CM-29 is used as the colored magenta coupler, can better prevent the variation between different types of printers, but seriously causes fog in both the fresh performance test and the raw storage stability test, and also shows a great decrease in sensitivity during raw storage. On the other hand, all the samples 3 to 18 in which the colored magenta coupler of the present invention and the heterocyclic ring containing fog restrainer are used in combination cause less fog in the fresh performance test and the raw storage stability test and also can much better prevent the variation between different types of between printers.
[0080] According to the present invention, it is possible to provide a light-sensitive silver halide color photographic material that may cause less increase in fog after storage when light-sensitive materials are stored in a high-temperature high-humidity environment, and may cause less variation between different types of printers used.
1. A light-sensitive silver halide color photographic material comprising a support and
provided thereon photographic component layers comprising a blue-sensitive silver
halide emulsion layer, a green-sensitive silver halide emulsion layer and a red-sensitive
silver halide emulsion layer, wherein at least one green-sensitive silver halide emulsion
layer contains a colored magenta coupler represented by the following Formula CM-1
and at least one of said photographic component layers contains a heterocyclic ring
containing fog restrainer:
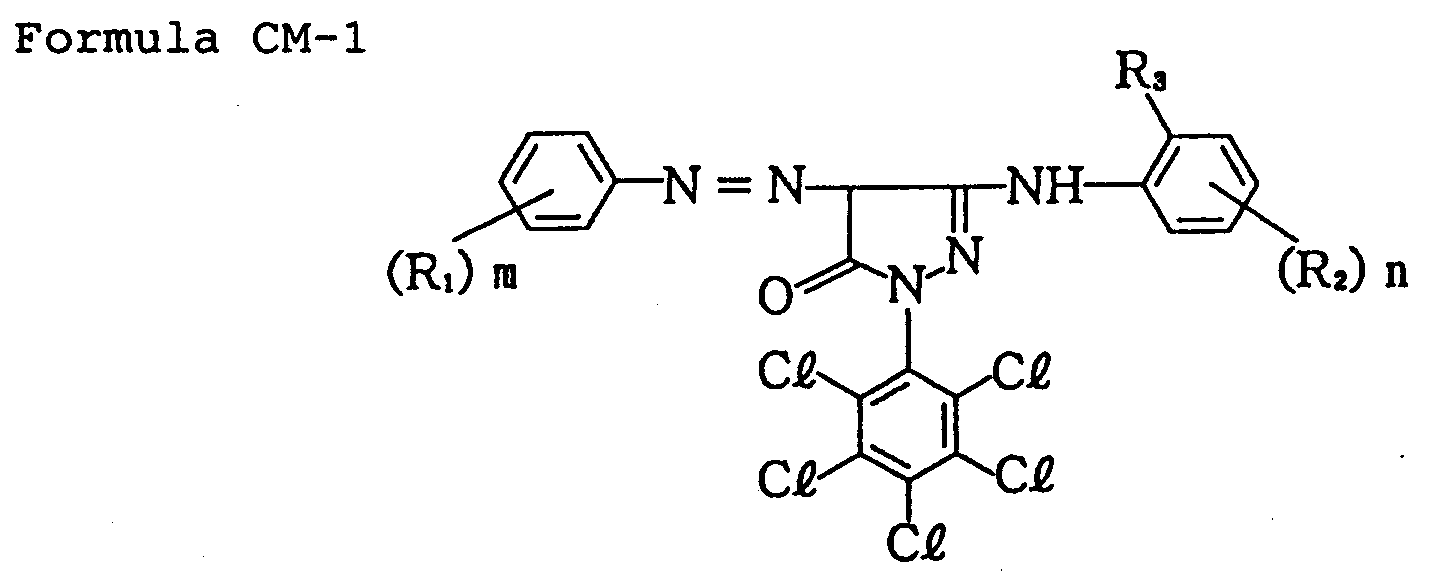
wherein R₁ represents a substituent; R₂ represents an acylamino group, a sulfonamide group, an imido group, a carbamoyl group, a sulfamoyl group, an alkoxy group, an alkoxycarbonyl group or an alkoxycarbonylamino group; R₃ represents a halogen atom or an alkoxy group; m represents an integer of 0 to 5; and n represents an integer of 0 to 4.
wherein R₁ represents a substituent; R₂ represents an acylamino group, a sulfonamide group, an imido group, a carbamoyl group, a sulfamoyl group, an alkoxy group, an alkoxycarbonyl group or an alkoxycarbonylamino group; R₃ represents a halogen atom or an alkoxy group; m represents an integer of 0 to 5; and n represents an integer of 0 to 4.
2. The material of claim 1, wherein said R₁ in Formula CM-1 includes an alkyl group,
an alkoxy group, an aryl group, an acylamino group, a sulfonamide group, a hydroxyl
group, a halogen atom, an alkoxycarbonyl group, an acyl group, a carbamoyl group,
a sulfamoyl group or a carboxyl group.
3. The material of claim 2, wherein said R₁ in Formula CM-1 includes an alkoxy group,
a hydroxyl group or an acylamino group.
4. The material of claim 1, wherein said R₂ in Formula CM-1 includes an acylamino group
substituted on the para position relative to R₃.
5. The material of claim 2, wherein said R₂ in Formula CM-1 includes an acylamino group
substituted on the para position relative to R₃.
6. The material of claim 3, wherein said R₂ in Formula CM-1 includes an acylamino group
substituted on the para position relative to R₃.
7. The material of claim 1, wherein said R₃ in Formula CM-1 includes a methoxy group
or a chlorine atom.
8. The material of claim 1, wherein said m in Formula CM-1 is 1 or 2, and said n in Formula
CM-1 is 1.
9. The material of claim 1, wherein said heterocyclic ring includes imidazoles, triazoles,
tetrazoles, thiadiazoles, oxadiazoles, pyridines, pyrimidines, benzimidazoles, benzotriazoles,
indazoles, benzotriazoles and azaindenes.
10. The material of claim 1, wherein said R₁ in Formula CM-1 represents an alkoxy group,
a hydroxyl group or an acylamino group; said R₂ in Formula CM-1 represents an acylamino
group substituted on the para position relative to R₃; said R₃ in Formula CM-1 represents
a chlorine atom; said m in Formula CM-1 is 1 or 2; and said n in Formula CM-1 is 1;
and said heterocyclic ring includes imidazoles, triazoles, tetrazoles, thiadiazoles,
oxadiazoles, pyridines, pyrimidines, benzimidazoles, benzotriazoles, indazoles, benzotriazoles
and azaindenes.
