|
(11) | EP 0 613 961 A1 |
| (12) | EUROPEAN PATENT APPLICATION |
|
|
|
|
|||||||||||||||||||||||||||
| (54) | Alloyed hot dip galvanized steel sheet |
| (57) An alloyed hot dip galvanized steel plate having high press workability and excellent
plating separation resistance. The alloyed hot dip galvanized steel plate is obtained
by forming an alloyed hot dip galvanized layer which contains 9 - 12 weight % of Fe
and 0.3 - 1.5 weight % of Al and about 0.1 weight % or less of Pb on the surface of
a ultra-low-carbon steel plate so that the amount of the adhesion thereof is from
25g/m² - 70g/m². |
BACKGROUND OF THE INVENTION
Field of the Invention
[0001] The present invention relates to an alloyed hot dip galvanized steel sheet which is used for external vehicle body plates having excellent pressing workability and plating separation resistance.
Description of Related Art
[0002] Recently, increased anticorrosive properties for vehicle bodies have been demanded. Industry has therefore been trying to develop adequate galvanized surface treatment steel sheets to meet these demands. One development has been hot dip galvanized steel sheets, which are economically excellent. Further, it has become possible to improve weldability and corrosion resistance after coating steel sheets by composing a plating layer of Fe-Zn (alloyed hot dip galvanized steel plate) and subjecting it to heat treatment.
[0003] Higher ductility (elongation El) and higher drawability (Lankford value, r value) have also been demanded because alloyed hot dip galvanized steel sheets used for external vehicle body sheets (hereinafter sometimes referred to as "GA") are subjected to high-level press processing to improve their design characteristics. To solve problems associated with achieving high ductility and high drawability, a variety of methods for reducing the content of C, N, P, S and the like contained in steels from the viewpoint of the property of the steel sheet as a material have been developed. Optimum hot rolling and cold rolling processes have also been developed.
[0004] In conjunction with the demands for the steel sheet, the characteristics demanded for the plating layer of GA steel sheets are that the plating layer must not exhibit 1) "powdering" in which the structure thereof is powdered and separated because it cannot follow the deformation of the steel sheet during processing, and 2) "flaking" in which the structure is separated in the form of scales when it is ironed by means of pressing dies. If such phenomena occur, separated plated particles are collected in the pressing dies, thereby causing highly undesirable indentations on the surface of steel sheets. Also, the corrosion resistance of the plating itself may be lost.
[0005] Generally, the plating layer of GA steel sheet is said to be composed of three Zn-Fe alloy phases ζ, δ₁, Γ from the viewpoint of low percentage content of Fe. The reason for powdering is believed to originate from the Γ phase and the reason for flaking is believed to originate from the ζ phase. If GA steel sheet is produced by hot-dip galvanizing the C, N, P, S reduced material, the ductility and r value are satisfied. However, if such a treatment is performed, alloying in the grain boundary of the steel sheet is excessively accelerated and increases the amount of Γ phase formation, thereby reducing powdering resistance. To secure a satisfactory powdering resistance, it is necessary to restrict the degree of alloying to the level (concentration of Fe) in which substantially no Γ phase is generated. However, in this case, an disclosed in Japanese Patent Laid-Open No.2-11745, if only the percentage content of Fe is restricted to cause no Γ phase, the ζ phase may form in a thick layer on the surface of the plating layer depending on production conditions, so that flaking becomes likely to occur when the layer is strongly flattened during pressing.
SUMMARY OF THE INVENTION
[0006] Accordingly, it is an object of the present invention to obtain an alloyed hot dip galvanized steel sheet in which the powdering resistance and flaking resistance can be satisfied at the same time by using a steel sheet obtained by reducing the content of C, N, P, S contained in the steel plate.
[0007] According to the present invention, there is provided an alloyed hot dip galvanized steel sheet having excellent press workability and plating separation resistance, wherein an alloyed hot dip galvanized layer which contains about 9 weight % or more and about 12 weight % or less of Fe, about 0.3 weight % or more and about 1.5 weight % or less of Al, about 0.1 weight % or less of Pb is formed on the surface of a steel sheet which contains about 0.0015 weight % or less of C, about 0.1 weight % or less of Si, about 0.03 weight % or more and about 0.3 weight % or less of Mn, about 0.01 weight % or more and about 0.1 weight % or less of Al, about 0.01 weight % or less of P, about 0.005 weight % or less of S, about 0.005 weight % or less of O, about 0.005 weight % or less of N and further contains at least one of about 0.03 weight % or less of Ti or about 0.03 weight % or less of Nb in the range in which
, the layer being in the thickness of about 25g/m² or more and about 70g/m² or less.
[0008] In this case, it is assumed that T* is
when
and Ti* is 0 when
. Additionally, the steel sheet having the aforementioned composition may contain about 0.001 weight % or less of B.
BRIEF DESCRIPTION OF THE DRAWINGS
DETAILED DESCRIPTION OF THE INVENTION
[0010] The alloyed hot dip galvanized steal sheet which has excellent press workability and plating separation resistance which is an object of the present invention will be described below. However, it will be appreciated that the following description is intended to refer to specific embodiments of the invention selected for illustration in the drawing and is not intended to define or limit the invention, other than in the appended claims.
[0011] First, the components contained in steel sheet which is to be used as the material for plating are determined as follows in order to satisfy necessary properties and achieve efficient economic production.
[0012] C: C is an element which directly determines the strength of steel. To obtain an extremely high workability (high El, r value) which is an object of the present invention, a smaller content thereof is better. The content thereof should be about 0.0015 weight % or less.
[0013] N, P and S: N, P and S are in the structure of steel to reduce the El and r values. As in the case of C, a smaller content of these elements is better. It is considered that N, P and S must be about 0.005 weight % or less, about 0.01 weight % or less, and about 0.005 weight % or less, respectively.
[0014] O: O is precipitated as an oxide if it is excessively contained in steel, thereby reducing the El and r values. Therefore, the content thereof must be about 0.005 weight % maximum.
[0015] Mn: If Mn is added into steel, it is combined with S and then precipitated to become non-effective. Thus, when a small amount of Mn is added, there is no remarkable effect from the viewpoint of the material. However, if the content thereof exceeds about 0.3 weight %, the El and r values decrease gradually. Thus, the content of Mn must be about 0.03 weight % or more and about 0.3 weight % or less.
[0016] Si: As in the case of Mn, a large amount of Si contained in steel reduces the El and r values and blocks the wettability of plating. Thus, the amount of Si is about 0.1 weight % maximum.
[0017] Ti and Nb: Ti and Nb are combined with C and precipitated in the form of TiC and NbC, thereby improving workability. Thus, the atomic ratio of Ti and Nb with respect to C needs to be more than about 1. However, because excessive addition of the elements increases cost, the maximum atomic ratio is about 6. Additionally, it is desirable that the maximum amount of each component is about 0.03 weight %. However, because Ti is more likely to be combined with N or S than C, it is necessary to determine the amount of Ti so that it is minus N and S equivalents. Specifically, it is assumed that the amounts of Ti and Nb satisfy the expression described below;
(where Ti* is
when
and Ti* is 0 when
)
Al: The amount of Al needs to be about 0.01 weight % or more to prevent Ti and Nb from being oxidized and lost when Ti and Nb are added. Al is combined with N and S contained in steel, thereby eliminating the effects thereof. However, if the amount of added Al exceeds about 0.1 weight %, the effect is saturated, so that excessive addition of Al is economically meaningless.
[0018] B: In the steel sheet according to the present invention, besides the basic composition described above, it is preferable that the amount of B is less than about 0.001 weight %. The reason is that B is effective in strengthening the grain boundary and improving spot weldability and secondary processing brittleness. However, if the amount of added B exceeds 0.001 weight %, the drawability is lost. Thus, the maximum amount is about 0.001 weight %.
[0020] The alloyed hot dip galvanized steel sheet is produced by immersing a steel sheet in a molten zinc bath and then heating the steel sheet to diffuse Fe contained in the steel sheet into plated layers, thereby forming a Zn-Fe alloy layer. Consequently, the corrosion resistance, chemical conversion treatment property and spot weldability thereof are markedly better than ordinary galvanized steel plates. These functions are preferably achieved by adjusting the content of Fe to about 9 weight % or more. Additionally, the amount of Fe needs to be about 9 weight % or more to prevent a ζ phase layer from growing. On the other hand, if the content of Fe exceeds about 12 weight %, a hard, brittle Γ phase layer is developed even if the content of Al in the plating layer is controlled in a range described later, thereby blocking press workability. Thus, the content of Fe contained in the plating layer must be about 9-12 weight % or less.
[0021] The content of Al contained in the plating layer affects the phase composition of the Zn-Fe alloy which is formed at the time of alloying. If the amount of Al is less than about 0.3 weight %, the Γ phase layer is developed so that undesirable powdering becomes likely. If the amount of Al exceeds about 1.5 weight %, insufficient alloying is achieved. Thus, the amount of Al contained in the plating layer is about 0.3 weight % - about 1.5 weight %.
[0022] Pb contained in the plating layer is restricted to about 0.2 weight % or less because it badly affects the corrosion resistance of the plating layer.
[0023] The amount of the plating layer applied needs to be about 25g/m² from the viewpoint of corrosion resistance. However, if the plating Layer is too thick, the layer cannot follow the deformation of the steel sheet when pressing is performed, thereby resulting in powdering. Thus, the maximum amount of plating applied to the steel plate is determined to be about 70g/cm².
[0024] Although the steel sheet production method according to the present invention is not particularly restricted to a specific method, a preferred production example will be described below.
[0025] Molten steel which is adjusted to the aforementioned composition is processed into a slab by means of a continuous casting method. The slab is then processed into cold finished steel plates through hot rolling and cold rolling. In hot rolling, it is desirable that the finishing temperature is about 850°C - 920°C which is near the Ar₃ transformation point to obtain high processing properties. It is desirable that the winding temperature is about 600°C or more. Further, in the cold rolling stop, it is desirable that the rolling reduction is about 50% or more.
[0026] In hot dip galvanizing, the surface of a steel sheet is purified before annealing reduction is performed. Degreasing, pickling or burning are permissible methods. The steel sheet is then subjected to annealing reduction. It is appropriate to use an H₂ atmosphere containing between several % and several tens % of N₂. It is also desirable that the dew point be 0°C or less. Although the annealing reduction temperature needs to be higher than the recrystallization temperature to secure a preferred material, it is desirable that the annealing reduction temperature is about 780°C or more.
[0027] After annealing reduction is performed, the steel sheet is cooled in reducing gas and introduced to a hot dip galvanizing bath. The components and the temperature of the bath are determined as follows.
[0028] Concentration of Al in the bath: One purpose of the present invention is to secure powdering resistance and flaking resistance by controlling the amount of the Al-Fe alloy layer generated in the galvanizing bath to achieve alloying of mainly δ₁ phase. The amount of the Al-Fe alloy layer should be adjusted so that the amount of Al contained in the alloy is 0.15g/m² or more for this purpose. Thus, the amount of Al in the bath needs to be about 0.13 weight % or more. To form the Al-Fe alloy layer effectively, it is desirable that the amount of Al in about 0.145 weight % or more. On the other hand, if the amount of the Al-Fe layer is increased so that the amount of Al exceeds about 0.5g/m², alloying is excessively restricted, so that productivity may be blocked. Namely, in the plating layer after alloying is performed, it is desirable that the amount of Al including Al contained in layers other than the Al-Fe layer is about 1.5 weight % at most. Thus, the maximum concentration of Al contained in the bath is about 0.2 weight %.
[0029] Concentration of Pb in the bath: Unlike Al, Pb in the bath is not concentrated on the plating during hot dipping. However, if the concentration of Pb in the plating layer exceeds about 0.1 weight %, corrosion resistance may drop. Thus, the upper limit of the concentration of Pb in the bath is about 0.1 weight %.
[0030] The steel sheet of the present invention can be used for various applications including automobiles, household electric appliances, construction materials, and the like in bare condition and/or in a condition which undergoes pre-coating, post-coating, laminating, chromate treatment, phosphate treatment or the like. Moreover, if the top layer of the alloyed hot dip galvanized plating layer is further coated with a plating layer containing at least one of Fe, Zn and Ni, the corrosion resistance is further improved.
[0031] After the steel sheet is immersed in the plating bath, it is subjected to alloying processing to obtain a GA steel sheet in which the degree of alloying (Fe) is 9 - 12%.
[0032] In the process described above, alloyed hot dip galvanized steel sheet which has excellent press processing and plating separation resistance can be obtained.
EXAMPLES:
[0033] Advantages of the present invention will be described with reference to examples thereof. Using a vertical type hot dip galvanizing experimental apparatus as a plating apparatus, a steel of 70mm x 200 mm was plated in an atmosphere containing an annealing reduction gas of 5%-hydrogen containing nitrogen. A heating oven which controlled the amount of heat generated by resistance by directly feeding power to a plated steel sheet was used for the alloying treatment of the plating.
[0034] A specimen of steel sheet was softened by means of a vacuum melting furnace, and hot rolled and cold rolled to adjust the thickness of the sheet to 0.7 mm. The sheet was subjected to electrolytic degreasing and pickling with hydrochloric acid before it was inserted into a plating apparatus. The hot rolling finish temperature was 900°C. After temporary cooling, the sheet was equally heated at 700°C for an hour according to the heat history obtained after it was coiled. The sheet was then cold rolled under a rolling reduction of 75% after it was cooled and pickled with acid.
[0035] Table 1 shows the components of the specimen steel sheet, the condition for plating and the composition of a plated layer provided before alloying treatment. Table 2 shows the characteristics of the plated steel sheet after the alloying treatment was performed. The material of the steel sheet was obtained by heat treatment according to CGL(Continuous Galvanizing Line) in an alloyed hot dip galvanizing cycle after which cold rolling was performed. The steel sheet was then annealed at 850°C for 20 seconds and cooled at 500°C for 30 seconds. Table 1 shows the components of the steel sheet as well.
[0036] The measurement of the amount of Al-Fe shown in Table 2 was performed by immersing a plated steel sheet before it was subjected to alloying processing in fuming nitric acid to remove zinc (η) phase, solving the Al-Fe alloy layer which was left unsolved in the passive state in hydrochloric acid and then measuring the amount of Al according to the atomic absorption method.
[0037] The elongation percentage (El) and r values of the steel sheet were obtained by tensile testing to evaluate the characteristics of the plated steel sheet. Powdering resistance and flaking resistance were obtained to investigate the characteristics of the plated layer. Powdering resistance was evaluated according to a five-step evaluation system by bending a plated steel sheet which had been subjected to alloying processing at 90 degrees, restoring it, and collecting separated plating particles using a preliminarily attached cellophane tape to measure the amount thereof. "1" indicated acceptable and "5" indicated unacceptable in the test.
[0038] Flaking resistance was measured by means of the bead type drawing test apparatus shown in Fig. 1 by using 10 mm wide cut pieces of the steel sheet which had undergone alloying processing. In the bead type drawing test apparatus, a test piece 2 was drawn through a bent path between an indented member 1 and a protruded member 3. The test piece coated with no oil was drawn under the condition in which the pressing load was 100kgf and that the drawing velocity was 500mm/min. Separated plated particles were collected using a cellophane tape to visually recognize whether flaking had occurred according to a two-level (yes/no) evaluation system.
[0039] As is evident from Tables 1 and 2, the present invention has succeeded in realizing production of alloyed hot dip galvanized steel sheet which has high workability and excellent plating separation resistance. Consequently, according to the present invention, it is possible to produce an alloyed hot dip galvanized steel sheet which has high workability and excellent plating separation resistance.
1. An alloyed hot dip galvanized steel sheet having excellent press workability and plating
separation resistance, wherein an alloyed hot dip galvanized layer containing about
9 weight % or more and about 12 weight % or less of Fe, about 0.3 weight % or more
and about 1.5 weight % or less of Al, and about 0.1 weight % or less of Pb is formed
on the surface of a steel plate containing about 0.0015 weight % or less of C, about
0.1 weight % or less of Si, about 0.03 weight % or more and about 0.3 weight % or
less of Mn, about 0.01 weight % or more and about 0.1 weight % or less of Al, about
0.01 weight % or less of P, about 0.005 weight % or less of S, about 0.005 weight
% or less of O, about 0.005 weight % or less of N, and at least one of about 0.03
weight % or less of Ti or about 0.03 weight % or less of Nb in the range in which
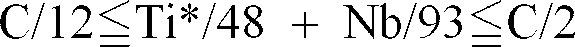
, the layer having a thickness of about 25g/m² or more and about 70g/m² or less; where T* is
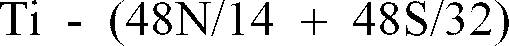
when
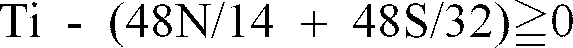
and Ti* is 0 when
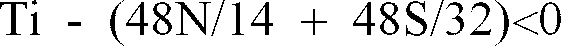
.
, the layer having a thickness of about 25g/m² or more and about 70g/m² or less; where T* is
when
and Ti* is 0 when
.
2. An alloyed hot dip galvanized steel sheet according to Claim 1, wherein said steel
plate further contains about 0.001 weight % or less of B, said steel plate having
excellent press workability and plating separation resistance.
3. An alloyed hot dip galvanized steel sheet having excellent press workability and plating
separation resistance comprising:
a steel plate containing about 0.0015 weight % or less of C, about 0.1 weight % or less of Si, about 0.03 weight % or more and about 0.3 weight % or less of Mn, about 0.01 weight % or more and about 0.1 weight % or less of Al, about 0.01 weight % or less of P, about 0.005 weight % or less of S, about 0 005 weight % or less of O, about 0.005 weight % or less of N, and at least one of about 0.03 weight % or less of Ti or about 0.03 weight % or less of Nb in the range in which
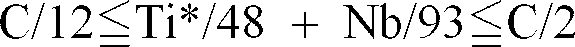
; and
an alloyed hot dip galvanized layer formed on said steel plate at a thickness of between about 25g/m² and about 70 g/m², said layer containing about 9 weight % or more and about 12 weight % or less of Fe, about 0.3 weight % or more and about 1.5 weight % or less of Al, and about 0.1 weight % or less of Pb.
a steel plate containing about 0.0015 weight % or less of C, about 0.1 weight % or less of Si, about 0.03 weight % or more and about 0.3 weight % or less of Mn, about 0.01 weight % or more and about 0.1 weight % or less of Al, about 0.01 weight % or less of P, about 0.005 weight % or less of S, about 0 005 weight % or less of O, about 0.005 weight % or less of N, and at least one of about 0.03 weight % or less of Ti or about 0.03 weight % or less of Nb in the range in which
; and
an alloyed hot dip galvanized layer formed on said steel plate at a thickness of between about 25g/m² and about 70 g/m², said layer containing about 9 weight % or more and about 12 weight % or less of Fe, about 0.3 weight % or more and about 1.5 weight % or less of Al, and about 0.1 weight % or less of Pb.
4. An alloyed hot dip galvanized steel sheet comprising:
a steel plate containing about 0.0015 weight % or less of C, about 0.1 weight % or less of Si, about 0.03 weight % to about 0.3 weight % of Mn, 0.01 weight % to about 0.1 weight % of Al, about 0.01 weight % or less of P, about 0.005 weight % or less of S, about 0.005 weight % or less of O, about 0.005 weight % or less of N, and at least one of about 0.03 weight % or less of Ti or about 0.03 weight % or less of Nb in the range in which
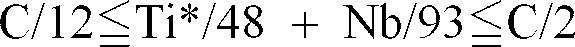
; and
an alloyed hot dip galvanized layer formed on said steel plate having a thickness of between about 25g/m² and about 70 g/m², said layer containing about 9 weight % to about 12 weight % of Fe, about 0.3 weight % to about 1.5 weight % of Al, and about 0.1 weight % or less of Pb.
a steel plate containing about 0.0015 weight % or less of C, about 0.1 weight % or less of Si, about 0.03 weight % to about 0.3 weight % of Mn, 0.01 weight % to about 0.1 weight % of Al, about 0.01 weight % or less of P, about 0.005 weight % or less of S, about 0.005 weight % or less of O, about 0.005 weight % or less of N, and at least one of about 0.03 weight % or less of Ti or about 0.03 weight % or less of Nb in the range in which
; and
an alloyed hot dip galvanized layer formed on said steel plate having a thickness of between about 25g/m² and about 70 g/m², said layer containing about 9 weight % to about 12 weight % of Fe, about 0.3 weight % to about 1.5 weight % of Al, and about 0.1 weight % or less of Pb.
